
Which of the following have asymmetric carbon atoms?
(A)
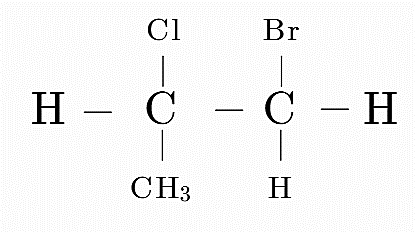
(B)
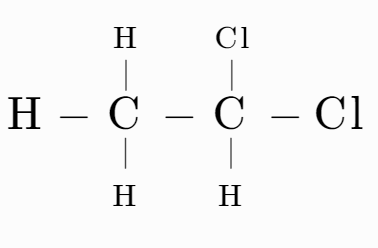
(C)
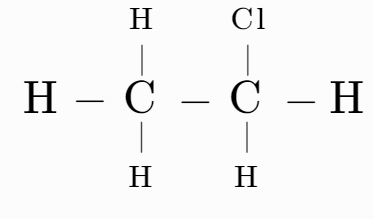
(D)
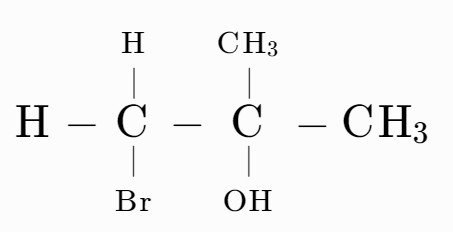




Answer
517.2k+ views
Hint :A chiral carbon atom (also known as an asymmetric carbon) is one that is bound to four distinct atoms or groups of atoms. There are two optical isomers for each chiral carbon in a molecule. In nature, only one optical isomer is always formed; for example, in translation, only L-isomer amino acids are produced.
Complete Step By Step Answer:
An asymmetric carbon atom (chiral carbon) is one that has four distinct forms of atoms or groups of atoms bound to it. The Le Bel-van't Hoff rule states that the number in stereoisomers of an organic compound is \[{2^n}\], where n is the number of asymmetric carbon atoms (unless there is an internal plane of symmetry); this is a corollary of Le Bel and van't Hoff's simultaneous announcements in 1874 that the most likely orientation of the bonds of a carbon atom linked to four groups of atoms is toward the apex (which involved a carbon atom bearing four different atoms or groups).
Consider option A
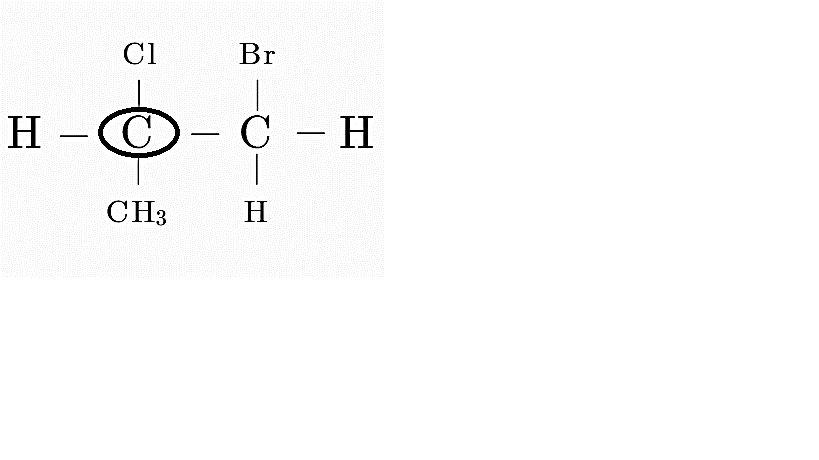
Here the first carbon is attached to 4 distinct groups, i.e, H, Cl, CH₃ and CH₂Br
Note :
Left-handed and right-handed representations of the same molecule result from the four sets of atoms bound to the carbon atom being arranged in space in two distinct forms that are mirror images of each other. Molecules that can't be superimposed over their own mirror image are chiral like mirror images.
Complete Step By Step Answer:
An asymmetric carbon atom (chiral carbon) is one that has four distinct forms of atoms or groups of atoms bound to it. The Le Bel-van't Hoff rule states that the number in stereoisomers of an organic compound is \[{2^n}\], where n is the number of asymmetric carbon atoms (unless there is an internal plane of symmetry); this is a corollary of Le Bel and van't Hoff's simultaneous announcements in 1874 that the most likely orientation of the bonds of a carbon atom linked to four groups of atoms is toward the apex (which involved a carbon atom bearing four different atoms or groups).
Consider option A

Here the first carbon is attached to 4 distinct groups, i.e, H, Cl, CH₃ and CH₂Br
Note :
Left-handed and right-handed representations of the same molecule result from the four sets of atoms bound to the carbon atom being arranged in space in two distinct forms that are mirror images of each other. Molecules that can't be superimposed over their own mirror image are chiral like mirror images.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




