
Which of the following ions has a square planar geometry?
(A) ${{[COC{{l}_{4}}]}^{2-}}$
(B) ${{[FeC{{l}_{4}}]}^{2-}}$
(C) ${{[NiC{{l}_{4}}]}^{2-}}$
(D) ${{[PtC{{l}_{4}}]}^{2-}}$
Answer
566.7k+ views
Hint: Square planar geometry is shown by the complex ions that show $ds{{p}^{2}}$ hybridization. Ions with coordination number 4 show this type of hybridization. In Square planar geometry all four hybrid orbitals together form a square.
Complete answer:
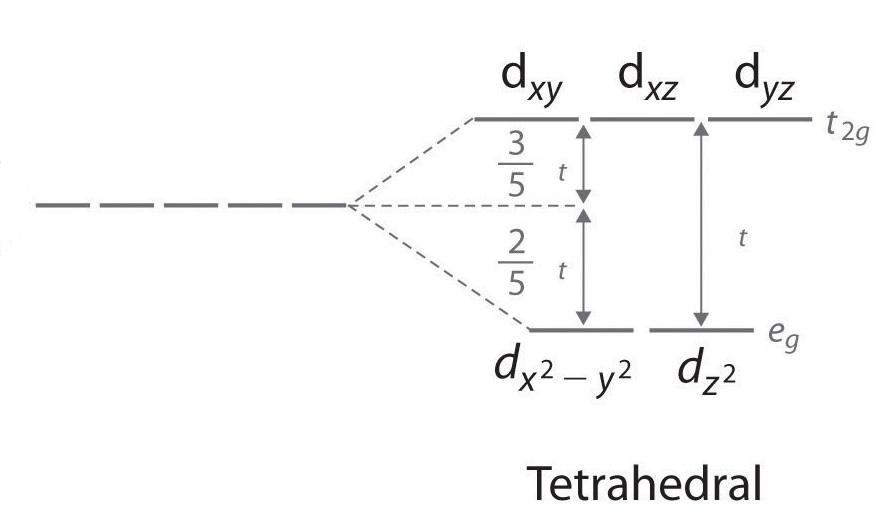
Tetrahedral crystal field splitting
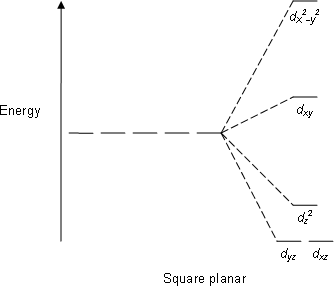
Square planar crystal field splitting:
To find out, square planar geometry let’s check each ions structure:
${{[COC{{l}_{4}}]}^{2-}}$: It has coordination number 4 and 4 weak field $C{{l}^{-}}$ ligands are attached to the cobalt ion.
Oxidation number of Co= charge on the ion- total charge on the ligand
Oxidation number of Co= -2-4(-1)
Oxidation number of Co=-2+4
Oxidation number of Co= +2
Electronic configuration of Cobalt atom: $[Ar]3{{d}^{7}}4{{s}^{2}}$
Electronic configuration of $C{{o}^{2+}}$= $[Ar]3{{d}^{7}}$
Now as the ligand will approach the metal ion, electron rearrangement will take place according to crystal field theory for weak field ligand.
Tetrahedral crystal field splitting will take place as shown in the diagram above and as cobalt is having 7 electrons for rearrangement, each d-orbital will have 1 electron first and then pairing of electrons will start.
Now here because Cobalt doesn’t have any empty d-orbital, therefore to take 4 lone pairs from ligands, it will use one 4s orbital and three 4p orbitals and here cobalt ion will have $s{{p}^{3}}$ hybridization and structure of the complex ion will be tetrahedral.
${{[FeC{{l}_{4}}]}^{2-}}$: It has coordination number 4 and 4 weak field $C{{l}^{-}}$ ligands are attached to the iron ion.
Oxidation number of Fe ion= charge on the ion- total charge on the ligand
Oxidation number of Fe= -2-4(-1)
Oxidation number of Fe=-2+4
Oxidation number of Fe= +2
Electronic configuration of Iron atom: $[Ar]3{{d}^{6}}4{{s}^{2}}$
Electronic configuration of $F{{e}^{2+}}$= $[Ar]3{{d}^{6}}$
Now as the ligand will approach the metal ion, electron rearrangement will take place according to crystal field theory for weak field ligand.
Tetrahedral crystal field splitting will take place as shown in the diagram above and as iron ions have 6 electrons for rearrangement, each d-orbital will have 1 electron first and then pairing of electrons will start.
Now here because Iron doesn’t have any empty d-orbital, therefore to take 4 lone pairs from ligands, it will use one 4s orbital and three 4p orbitals and here iron ion will have $s{{p}^{3}}$hybridization and structure of the complex ion will be tetrahedral.
${{[NiC{{l}_{4}}]}^{2-}}$: It has coordination number 4 and 4 weak field $C{{l}^{-}}$ ligands are attached to the Nickel ion.
Oxidation number of Ni ion= charge on the ion- total charge on the ligand
Oxidation number of Ni= -2-4(-1)
Oxidation number of Ni=-2+4
Oxidation number of Ni= +2
Electronic configuration of Nickel atom: $[Ar]3{{d}^{8}}4{{s}^{2}}$
Electronic configuration of $N{{i}^{2+}}$= $[Ar]3{{d}^{8}}$
Now as the ligand will approach the metal ion, electron rearrangement will take place according to crystal field theory for weak field ligand.
Tetrahedral crystal field splitting will take place as shown in the diagram above and as nickel ions have 8 electrons for rearrangement, each d-orbital will have 1 electron first and then pairing of electrons will start.
Now here because Iron doesn’t have any empty d-orbital, therefore to take 4 lone pairs from ligands, it will use one 4s orbital and three 4p orbitals and here nickel ion will have $s{{p}^{3}}$ hybridization and structure of the complex ion will be tetrahedral.
\[{{[PtC{{l}_{4}}]}^{2-}}\]: It has coordination number 4 and 4 weak field $C{{l}^{-}}$ ligands are attached to the platinum ion.
Oxidation number of Pt ion= charge on the ion- total charge on the ligand
Oxidation number of Pt= -2-4(-1)
Oxidation number of Pt=-2+4
Oxidation number of Pt= +2
Electronic configuration of Pt atom: $[Xe]4{{f}^{14}}5{{d}^{9}}6{{s}^{1}}$
Electronic configuration of $P{{t}^{2+}}$= $[Xe]4{{f}^{14}}5{{d}^{8}}$
Now as the ligand will approach the metal ion, electron rearrangement will take place according to crystal field theory.
Square planar crystal field splitting will take place as shown in the diagram above and as platinum ion is having 8 electrons for rearrangement, electron rearrangement will be done according to energy gap and therefore four d orbitals will have 8 electrons and one d-orbital which is having highest energy will remain empty. Now here because Pt does have empty d-orbital, therefore to take 4 lone pairs from ligands, it will use one 5d orbital, one 6s orbital and two 6p orbitals and here platinum ion will have $ds{{p}^{2}}$ hybridization and structure of the complex ion will be Square planar.
So correct answer is Option (D)
Additional information: There are two types of complexes, one is high spinning complex and other is low spin complex. When a central metal ion is having quite a few unpaired electrons then it will be a high spin complex and when it has zero or very few unpaired electrons then it will be a low spin complex.
Note: Spectrochemical series is the important series that help us to decide the strength of ligands. Spectrochemical series is the important series that help us to decide the strength of ligands . According to that generally the ligands where the donor atom has the high electronegativity is a weak field ligand and generally the ligands like cyanide ion, CO etc. where donor atom is carbon are strong field ligands.
Complete answer:
Tetrahedral crystal field splitting

Square planar crystal field splitting:

To find out, square planar geometry let’s check each ions structure:
${{[COC{{l}_{4}}]}^{2-}}$: It has coordination number 4 and 4 weak field $C{{l}^{-}}$ ligands are attached to the cobalt ion.
Oxidation number of Co= charge on the ion- total charge on the ligand
Oxidation number of Co= -2-4(-1)
Oxidation number of Co=-2+4
Oxidation number of Co= +2
Electronic configuration of Cobalt atom: $[Ar]3{{d}^{7}}4{{s}^{2}}$
Electronic configuration of $C{{o}^{2+}}$= $[Ar]3{{d}^{7}}$
Now as the ligand will approach the metal ion, electron rearrangement will take place according to crystal field theory for weak field ligand.
Tetrahedral crystal field splitting will take place as shown in the diagram above and as cobalt is having 7 electrons for rearrangement, each d-orbital will have 1 electron first and then pairing of electrons will start.
Now here because Cobalt doesn’t have any empty d-orbital, therefore to take 4 lone pairs from ligands, it will use one 4s orbital and three 4p orbitals and here cobalt ion will have $s{{p}^{3}}$ hybridization and structure of the complex ion will be tetrahedral.
${{[FeC{{l}_{4}}]}^{2-}}$: It has coordination number 4 and 4 weak field $C{{l}^{-}}$ ligands are attached to the iron ion.
Oxidation number of Fe ion= charge on the ion- total charge on the ligand
Oxidation number of Fe= -2-4(-1)
Oxidation number of Fe=-2+4
Oxidation number of Fe= +2
Electronic configuration of Iron atom: $[Ar]3{{d}^{6}}4{{s}^{2}}$
Electronic configuration of $F{{e}^{2+}}$= $[Ar]3{{d}^{6}}$
Now as the ligand will approach the metal ion, electron rearrangement will take place according to crystal field theory for weak field ligand.
Tetrahedral crystal field splitting will take place as shown in the diagram above and as iron ions have 6 electrons for rearrangement, each d-orbital will have 1 electron first and then pairing of electrons will start.
Now here because Iron doesn’t have any empty d-orbital, therefore to take 4 lone pairs from ligands, it will use one 4s orbital and three 4p orbitals and here iron ion will have $s{{p}^{3}}$hybridization and structure of the complex ion will be tetrahedral.
${{[NiC{{l}_{4}}]}^{2-}}$: It has coordination number 4 and 4 weak field $C{{l}^{-}}$ ligands are attached to the Nickel ion.
Oxidation number of Ni ion= charge on the ion- total charge on the ligand
Oxidation number of Ni= -2-4(-1)
Oxidation number of Ni=-2+4
Oxidation number of Ni= +2
Electronic configuration of Nickel atom: $[Ar]3{{d}^{8}}4{{s}^{2}}$
Electronic configuration of $N{{i}^{2+}}$= $[Ar]3{{d}^{8}}$
Now as the ligand will approach the metal ion, electron rearrangement will take place according to crystal field theory for weak field ligand.
Tetrahedral crystal field splitting will take place as shown in the diagram above and as nickel ions have 8 electrons for rearrangement, each d-orbital will have 1 electron first and then pairing of electrons will start.
Now here because Iron doesn’t have any empty d-orbital, therefore to take 4 lone pairs from ligands, it will use one 4s orbital and three 4p orbitals and here nickel ion will have $s{{p}^{3}}$ hybridization and structure of the complex ion will be tetrahedral.
\[{{[PtC{{l}_{4}}]}^{2-}}\]: It has coordination number 4 and 4 weak field $C{{l}^{-}}$ ligands are attached to the platinum ion.
Oxidation number of Pt ion= charge on the ion- total charge on the ligand
Oxidation number of Pt= -2-4(-1)
Oxidation number of Pt=-2+4
Oxidation number of Pt= +2
Electronic configuration of Pt atom: $[Xe]4{{f}^{14}}5{{d}^{9}}6{{s}^{1}}$
Electronic configuration of $P{{t}^{2+}}$= $[Xe]4{{f}^{14}}5{{d}^{8}}$
Now as the ligand will approach the metal ion, electron rearrangement will take place according to crystal field theory.
Square planar crystal field splitting will take place as shown in the diagram above and as platinum ion is having 8 electrons for rearrangement, electron rearrangement will be done according to energy gap and therefore four d orbitals will have 8 electrons and one d-orbital which is having highest energy will remain empty. Now here because Pt does have empty d-orbital, therefore to take 4 lone pairs from ligands, it will use one 5d orbital, one 6s orbital and two 6p orbitals and here platinum ion will have $ds{{p}^{2}}$ hybridization and structure of the complex ion will be Square planar.
So correct answer is Option (D)
Additional information: There are two types of complexes, one is high spinning complex and other is low spin complex. When a central metal ion is having quite a few unpaired electrons then it will be a high spin complex and when it has zero or very few unpaired electrons then it will be a low spin complex.
Note: Spectrochemical series is the important series that help us to decide the strength of ligands. Spectrochemical series is the important series that help us to decide the strength of ligands . According to that generally the ligands where the donor atom has the high electronegativity is a weak field ligand and generally the ligands like cyanide ion, CO etc. where donor atom is carbon are strong field ligands.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




