
Which of the following is a geminal dihalide?
A. Ethylene dibromide
B. Propylidene chloride
C. Isopropyl bromide
D. None of the above.
Answer
570.9k+ views
Hint: The answer here is based on the concept of inorganic chemistry that deals with the fact that germinal means relationship between two atoms or functional groups that are attached to the same atom and by writing the structures of given compound, you get the correct answer.
Complete answer:
We have come across the concepts of inorganic chemistry that deals with the part about the geminal halides and their properties.
We shall refresh the concept and shall deduce the structures of given compounds that leads us to the required answer.
- Geminal dihalides are those compounds where both the halide groups are attached to the same carbon atom.
- To deduce the correct answer, let us draw the structures of the given molecules and then understand which is the geminal dihalide.
In the option A. Ethylene dibromide that is 1,2 - dibromoethane the structure is as shown below,
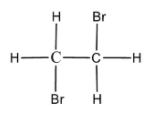
Here, the bromine atoms are attached to the different carbon atoms and thus is not a geminal dihalide.
In option B. Propylidene chloride also called as 1,1 - dichloropropane
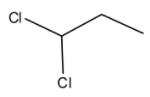
Since, the chlorine is attached to the same carbon atom and thus this compound is a geminal dihalide.
In option C. Isopropyl bromide also known as 2 – bromopropane has the following structure,
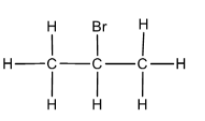
Since, there is a single bromine atom attached to a single carbon atom the compound is not a geminal dihalide.
Therefore, the correct answer is option B. Propylidene chloride.
Note:
Note that there is also a term called vicinal dihalides and do not be confused as vicinal dihalides are those compounds that have the halogens present on the adjacent carbon and these are prepared by the reaction between a halogen and an alkene.
Complete answer:
We have come across the concepts of inorganic chemistry that deals with the part about the geminal halides and their properties.
We shall refresh the concept and shall deduce the structures of given compounds that leads us to the required answer.
- Geminal dihalides are those compounds where both the halide groups are attached to the same carbon atom.
- To deduce the correct answer, let us draw the structures of the given molecules and then understand which is the geminal dihalide.
In the option A. Ethylene dibromide that is 1,2 - dibromoethane the structure is as shown below,

Here, the bromine atoms are attached to the different carbon atoms and thus is not a geminal dihalide.
In option B. Propylidene chloride also called as 1,1 - dichloropropane

Since, the chlorine is attached to the same carbon atom and thus this compound is a geminal dihalide.
In option C. Isopropyl bromide also known as 2 – bromopropane has the following structure,

Since, there is a single bromine atom attached to a single carbon atom the compound is not a geminal dihalide.
Therefore, the correct answer is option B. Propylidene chloride.
Note:
Note that there is also a term called vicinal dihalides and do not be confused as vicinal dihalides are those compounds that have the halogens present on the adjacent carbon and these are prepared by the reaction between a halogen and an alkene.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




