
Which of the following is active species in sulphonation of benzene?
a.) ${ SO }_{ 3 }$
b.) ${ { SO }_{ 3 } }^{ + }$
c.) ${ { SO }_{ 3 } }^{ - }$
d.) ${ { HSO }_{ 4 } }^{ - }$
Answer
588k+ views
Hint: You should know that this chemical compound occurs in the gaseous form and it is a primary agent in acid rain. It is generally prepared on an industrial scale as a precursor to sulfuric acid. It is also known as sulfuric anhydride.
Complete step by step answer:
To solve this, first let us understand about the Sulphonation reaction.
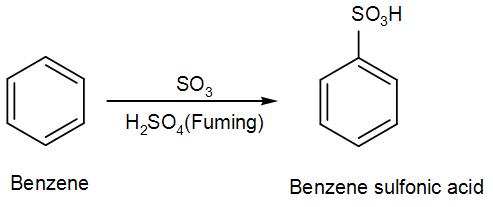
Sulphonation is generally a reversible reaction that produces benzenesulfonic acid by adding sulfur trioxide and fuming sulfuric acid. The reaction is generally reversed by adding hot aqueous acid to benzenesulfonic acid to produce benzene.
Now, let us understand the mechanism in simple words.
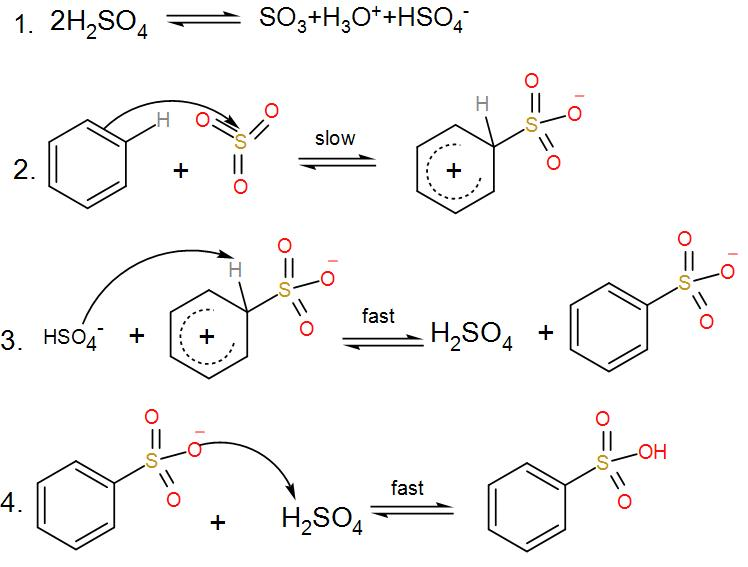
- Firstly, from sulphuric acid we get sulphur trioxide.
- To produce benzenesulfonic acid from benzene, fuming sulfuric acid and sulfur trioxide are added.
- Fuming sulfuric acid, also called oleum, is a concentrated solution of dissolved sulfur trioxide in sulfuric acid.
- The sulfur in sulfur trioxide is electrophilic because the oxygen pulls the electrons away from it because as we know oxygen is very electronegative in nature.
- Now the benzene attacks the sulfur to produce benzenesulfonic acid.
Now let us also understand about reverse sulfonation.
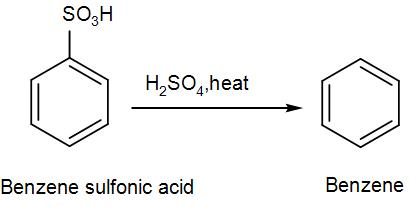
Sulphonation of benzene is a reversible reaction. Sulfur trioxide readily reacts with water to produce sulfuric acid and heat. Therefore, by adding heat to benzenesulfonic acid in diluted aqueous sulfuric acid the reaction is reversed.
We can understand from the above discussions that sulphur trioxide acts as an electrophile and accepts a pair of –pi electrons from benzene and thus moves the reaction forward.
Therefore, the correct answer is ${ SO }_{ 3 }$
So, the correct answer is “Option A”.
Note: As we have already seen sulfur trioxide is used as an intermediate in the production of sulfuric acid and other chemicals. You should know that ingestion causes severe burns of mouth and stomach. So be careful!
Complete step by step answer:
To solve this, first let us understand about the Sulphonation reaction.
Sulphonation is generally a reversible reaction that produces benzenesulfonic acid by adding sulfur trioxide and fuming sulfuric acid. The reaction is generally reversed by adding hot aqueous acid to benzenesulfonic acid to produce benzene.

Now, let us understand the mechanism in simple words.
- Firstly, from sulphuric acid we get sulphur trioxide.
- To produce benzenesulfonic acid from benzene, fuming sulfuric acid and sulfur trioxide are added.
- Fuming sulfuric acid, also called oleum, is a concentrated solution of dissolved sulfur trioxide in sulfuric acid.
- The sulfur in sulfur trioxide is electrophilic because the oxygen pulls the electrons away from it because as we know oxygen is very electronegative in nature.
- Now the benzene attacks the sulfur to produce benzenesulfonic acid.

Now let us also understand about reverse sulfonation.
Sulphonation of benzene is a reversible reaction. Sulfur trioxide readily reacts with water to produce sulfuric acid and heat. Therefore, by adding heat to benzenesulfonic acid in diluted aqueous sulfuric acid the reaction is reversed.

We can understand from the above discussions that sulphur trioxide acts as an electrophile and accepts a pair of –pi electrons from benzene and thus moves the reaction forward.
Therefore, the correct answer is ${ SO }_{ 3 }$
So, the correct answer is “Option A”.
Note: As we have already seen sulfur trioxide is used as an intermediate in the production of sulfuric acid and other chemicals. You should know that ingestion causes severe burns of mouth and stomach. So be careful!
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




