
Which of the following is least stable
A. $C{{H}_{3}}-C{{H}^{+}}-C{{H}_{3}}$
B. $C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}^{+}$
C.
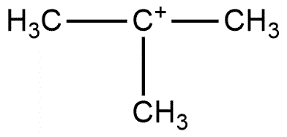
D.



Answer
552.3k+ views
Hint: A carbocation is those substances in which a carbon atom has a positive charge also we can consider that carbocation is made up of two words carbo and cation where carbo is for carbon and cation is positively charged ions.
Complete step by step answer:
The stability of the carbocation is generally dependent upon the resonance, inductive, or hyperconjugation effect and electronegativity value of the compound which can be explained as stability of the carbocation increases with the increase in the number of resonating structures this is due to the delocalization of positive charge. Hyper conjugation is increased as we increase the substitution in carbocations and we can say that tertiary carbocation is more stable as compared to secondary and primary. Electronegativity tells about the capacity of an atom to attract electrons. As the electronegativity of the carbon atom increases the stability of carbocation decreases.
Out of all the examples, we can see that option B has the least number of alpha hydrogens so it is termed as least stable and alpha hydrogens are also absent in case D but resonance will occur in this case. So, the correct answer is “Option B”.
Note: Carbocations are also known by the name carbonium ion. Examples given in the question are carbocation and these types of compounds are very reactive due to their incomplete octet i.e. they do not have 8 electrons in their outermost shell so they are not able to follow the octet rule.
Complete step by step answer:
The stability of the carbocation is generally dependent upon the resonance, inductive, or hyperconjugation effect and electronegativity value of the compound which can be explained as stability of the carbocation increases with the increase in the number of resonating structures this is due to the delocalization of positive charge. Hyper conjugation is increased as we increase the substitution in carbocations and we can say that tertiary carbocation is more stable as compared to secondary and primary. Electronegativity tells about the capacity of an atom to attract electrons. As the electronegativity of the carbon atom increases the stability of carbocation decreases.
Out of all the examples, we can see that option B has the least number of alpha hydrogens so it is termed as least stable and alpha hydrogens are also absent in case D but resonance will occur in this case. So, the correct answer is “Option B”.
Note: Carbocations are also known by the name carbonium ion. Examples given in the question are carbocation and these types of compounds are very reactive due to their incomplete octet i.e. they do not have 8 electrons in their outermost shell so they are not able to follow the octet rule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




