
Which of the following is most reactive towards nucleophilic addition
A. Acetaldehyde
B. Formaldehyde
C. Acetone
D. Benzophenone
Answer
527.4k+ views
Hint:
As we know that depending upon the nature of the attacking species, addition reactions can be nucleophilic, electrophilic and free radical addition reactions. Nucleophilic reactions are typical of aldehydes and ketones and aldehydes are more reactive towards it due to their electronic reasons and steric hindrance.
Complete answer:
As we all know that depending upon the nature of the attacking species, additional reactions can be nucleophilic, electrophilic and free radical addition reactions. Nucleophilic reactions are typical of aldehydes and ketones and aldehydes are more reactive towards it due to their electronic reasons and steric hindrance. We can say that Aldehydes are more reactive than ketones because in ketones, the large substituents will hinder the approach of nucleophiles to the carbonyl carbon.
When a nucleophile attacks the aldehyde or ketone carbon, the incoming nucleophile pushes the electrons in the pi bond on to the oxygen, carbon becomes partially positive and oxygen partially negative. So now the nucleophile attacks the carbon and donate its electron and the oxygen has the ability to act as a nucleophile or base and abstract a proton from the acid group present nearby.
We can represent this reaction through a diagram:
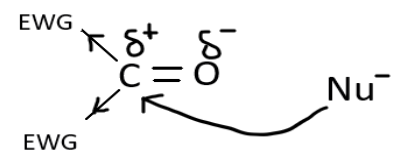
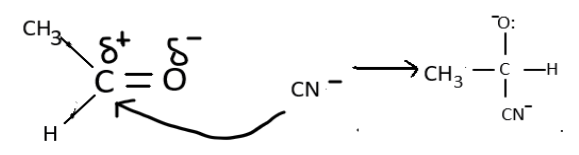
Similarly, we can depict the nucleophilic addition in acetaldehyde which results in cyanohydrin formation which on further hydrolysis forms an alpha hydroxy acid.
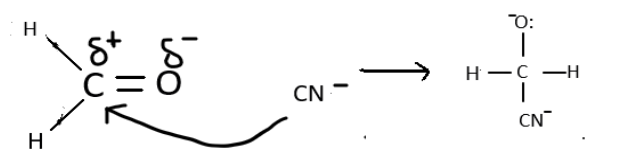
Formaldehyde will reacts in the same and form a cyanohydrin as depicted:
Now out of these two, we can see that acetaldehyde possess a methyl group which decreases the reactivity of the molecule by decreasing its electron deficiency, and we also know that acetone have high steric hindrance and two methyl groups and greater –inductive effect and benzophenone being an aromatic ketone will be bulky.
Hence, from the above equation we can say that Formaldehyde is more reactive towards nucleophilic addition reaction.
Therefore, the correct answer is B. Formaldehyde
Note:
Some interesting points about formaldehyde: formaldehyde is stored as formalin due to the pungent smelling gas which spontaneously polymerises into paraformaldehyde that is insoluble in most of the solvents. Its aqueous solution is used as a disinfectant to kill bacteria and fungi.
As we know that depending upon the nature of the attacking species, addition reactions can be nucleophilic, electrophilic and free radical addition reactions. Nucleophilic reactions are typical of aldehydes and ketones and aldehydes are more reactive towards it due to their electronic reasons and steric hindrance.
Complete answer:
As we all know that depending upon the nature of the attacking species, additional reactions can be nucleophilic, electrophilic and free radical addition reactions. Nucleophilic reactions are typical of aldehydes and ketones and aldehydes are more reactive towards it due to their electronic reasons and steric hindrance. We can say that Aldehydes are more reactive than ketones because in ketones, the large substituents will hinder the approach of nucleophiles to the carbonyl carbon.
When a nucleophile attacks the aldehyde or ketone carbon, the incoming nucleophile pushes the electrons in the pi bond on to the oxygen, carbon becomes partially positive and oxygen partially negative. So now the nucleophile attacks the carbon and donate its electron and the oxygen has the ability to act as a nucleophile or base and abstract a proton from the acid group present nearby.
We can represent this reaction through a diagram:
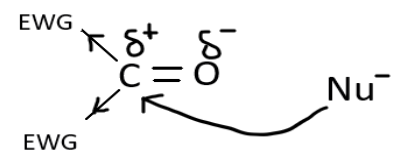
Similarly, we can depict the nucleophilic addition in acetaldehyde which results in cyanohydrin formation which on further hydrolysis forms an alpha hydroxy acid.

Formaldehyde will reacts in the same and form a cyanohydrin as depicted:

Now out of these two, we can see that acetaldehyde possess a methyl group which decreases the reactivity of the molecule by decreasing its electron deficiency, and we also know that acetone have high steric hindrance and two methyl groups and greater –inductive effect and benzophenone being an aromatic ketone will be bulky.
Hence, from the above equation we can say that Formaldehyde is more reactive towards nucleophilic addition reaction.
Therefore, the correct answer is B. Formaldehyde
Note:
Some interesting points about formaldehyde: formaldehyde is stored as formalin due to the pungent smelling gas which spontaneously polymerises into paraformaldehyde that is insoluble in most of the solvents. Its aqueous solution is used as a disinfectant to kill bacteria and fungi.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




