
Which of the following is not an isomer of Butanal?
(A)2-Butanone
(B)2-methyl propanal
(C)2-Butanol
(D)But-2-en-1-ol
Answer
586.5k+ views
Hint: Isomers can be identified on the basis of molecular formula. Molecules with different functional groups can also be isomers of each other. Spatial arrangement of the two molecules which are isomers will be different.
Complete answer:
Butanal is four carbon containing hydrocarbon with aldehyde functional group present.The molecular formula of butanal is ${{C}_{4}}{{H}_{8}}O$ .The structure of butanal is as follows:
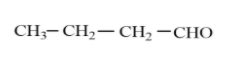
Isomers are those molecules which have the same molecular formula, that same number of atoms of each element, with different spatial arrangement. To see which of the options is not the correct isomer of butanal, we will see the structure and molecular formula of each of the options given in the question.
Considering option (A), 2-Butanone. The molecular formula of 2-Butanone is ${{C}_{4}}{{H}_{8}}O$. The structure of 2-Butanone is given as follows:
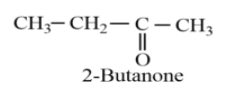
The molecular formula of 2-butanone is same as that of butanal, but is with different spatial arrangement, therefore, it is the isomer of butanal.
Considering option (B), 2-methyl propanal. The molecular formula of 2-methyl propanal is ${{C}_{4}}{{H}_{8}}O$. The structure of 2-methyl propanal is given as follows:
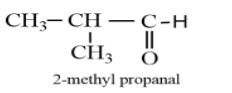
The molecular formula of 2-methyl propanal is same as that of butanal, but is with different spatial arrangement, therefore, it is the isomer of butanal.
Considering option (C), 2-Butanol. The molecular formula of 2-Butanol is ${{C}_{4}}{{H}_{10}}O$. The structure of is 2-Butanol given as follows:
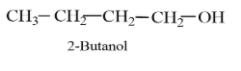
The molecular formula of 2-butanol is not the same as that of butanal, therefore, it is not an isomer of butanal.
Considering option (D), But-2-en-1-ol. The molecular formula of But-2-en-1-ol is ${{C}_{4}}{{H}_{8}}O$. The structure of But-2-en-1-ol is given as follows:
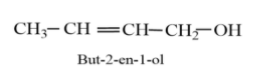
The molecular formula of But-2-en-1-ol is same as that of butanal, but is with different spatial arrangement, therefore, it is the isomer of butanal.
Therefore, the correct answer is option (C).
Note:
There are different types of isomers. Like, structural isomers. But-2-en-1-ol, 2-methyl propanal, 2-Butanone are structural isomers of butanal. However , other types of isomers are chain isomers, position isomers,functional group isomers,hydration isomers, linkage isomers.
Complete answer:
Butanal is four carbon containing hydrocarbon with aldehyde functional group present.The molecular formula of butanal is ${{C}_{4}}{{H}_{8}}O$ .The structure of butanal is as follows:
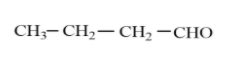
Isomers are those molecules which have the same molecular formula, that same number of atoms of each element, with different spatial arrangement. To see which of the options is not the correct isomer of butanal, we will see the structure and molecular formula of each of the options given in the question.
Considering option (A), 2-Butanone. The molecular formula of 2-Butanone is ${{C}_{4}}{{H}_{8}}O$. The structure of 2-Butanone is given as follows:

The molecular formula of 2-butanone is same as that of butanal, but is with different spatial arrangement, therefore, it is the isomer of butanal.
Considering option (B), 2-methyl propanal. The molecular formula of 2-methyl propanal is ${{C}_{4}}{{H}_{8}}O$. The structure of 2-methyl propanal is given as follows:
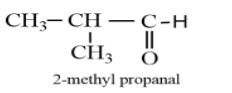
The molecular formula of 2-methyl propanal is same as that of butanal, but is with different spatial arrangement, therefore, it is the isomer of butanal.
Considering option (C), 2-Butanol. The molecular formula of 2-Butanol is ${{C}_{4}}{{H}_{10}}O$. The structure of is 2-Butanol given as follows:
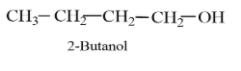
The molecular formula of 2-butanol is not the same as that of butanal, therefore, it is not an isomer of butanal.
Considering option (D), But-2-en-1-ol. The molecular formula of But-2-en-1-ol is ${{C}_{4}}{{H}_{8}}O$. The structure of But-2-en-1-ol is given as follows:
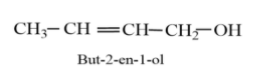
The molecular formula of But-2-en-1-ol is same as that of butanal, but is with different spatial arrangement, therefore, it is the isomer of butanal.
Therefore, the correct answer is option (C).
Note:
There are different types of isomers. Like, structural isomers. But-2-en-1-ol, 2-methyl propanal, 2-Butanone are structural isomers of butanal. However , other types of isomers are chain isomers, position isomers,functional group isomers,hydration isomers, linkage isomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




