
Which of the following is the separation technique to separate mercury and water?
(A) separating funnel
(B) sublimation
(C) filtration followed by evaporation
(D) separating funnel followed by evaporation or distillation.
Answer
588.9k+ views
Hint: The various components of a mixture of two liquids have different physical properties such as density, solubility, size of particles, volatility, boiling point, and melting point, etc. there are different methods to separate the mixture of two liquids that are miscible or immiscible between them.
Complete step by step solution:
The physical properties of liquids in a mixture, the separation techniques differ from miscible liquids from immiscible liquids. Miscible liquid means the two liquids are completely soluble with each other. Immiscible liquids are not soluble completely with each other.
The method is used to separate immiscible liquids like mercury and water is the decantation separating technique.
Decantation: this is the process used to the mixture of solid in liquid or two immiscible liquids based on their difference in density. The mixture to be separated is gradually poured from one container to another or using a separating funnel.
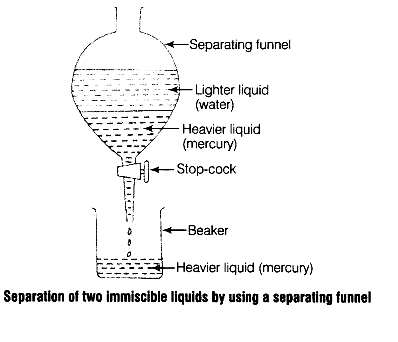
In the given mixture of mercury and water, mercury is denser than water. So, during the separation of this mixture using the decantation technique in a separating funnel, mercury forms the lower layer and separates from the water as shown in the above diagram.
so, the separation technique to separate mercury and water is separating funnels.
The correct answer is option A.
Note: There are many techniques used to separate miscible liquids in a mixture based on their physical properties. For example, the separating mixture of two or more liquids by fraction distillation process based on their boiling points. In this process, the highest boiling comes first and after the next one. Separating the mixtures in petroleum by using this technique is called fractional distillation of petroleum.
Complete step by step solution:
The physical properties of liquids in a mixture, the separation techniques differ from miscible liquids from immiscible liquids. Miscible liquid means the two liquids are completely soluble with each other. Immiscible liquids are not soluble completely with each other.
The method is used to separate immiscible liquids like mercury and water is the decantation separating technique.
Decantation: this is the process used to the mixture of solid in liquid or two immiscible liquids based on their difference in density. The mixture to be separated is gradually poured from one container to another or using a separating funnel.

In the given mixture of mercury and water, mercury is denser than water. So, during the separation of this mixture using the decantation technique in a separating funnel, mercury forms the lower layer and separates from the water as shown in the above diagram.
so, the separation technique to separate mercury and water is separating funnels.
The correct answer is option A.
Note: There are many techniques used to separate miscible liquids in a mixture based on their physical properties. For example, the separating mixture of two or more liquids by fraction distillation process based on their boiling points. In this process, the highest boiling comes first and after the next one. Separating the mixtures in petroleum by using this technique is called fractional distillation of petroleum.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




