
Which of the following pair is \[{C_2}\] epimer:
A.D-Glucose, D-Maltose
B.D-Glucose, D-Mannose
C.D-Allose, D-Ribose
D.D-Glucose, D-Arabinose
Answer
585.6k+ views
Hint: Epimer in stereochemistry describes one of a pair of stereoisomers. At the stereo genic centre, two isomers present in the molecules differ, while the rest remains the same. A molecule can have various stereo enters leading to several stereo enters.
Complete step by step answer:
Epimers are the diastereomers that contain one or more chiral carbon but differ in their configuration at only one chiral carbon in their structures. Let us look at the structure of D-Glucose, the carbon chain is assigned numbers as per functional group priority. At its C2 position, the constituents are exchanged from right to left to left to right. This gives a new compound called D-Mannose.
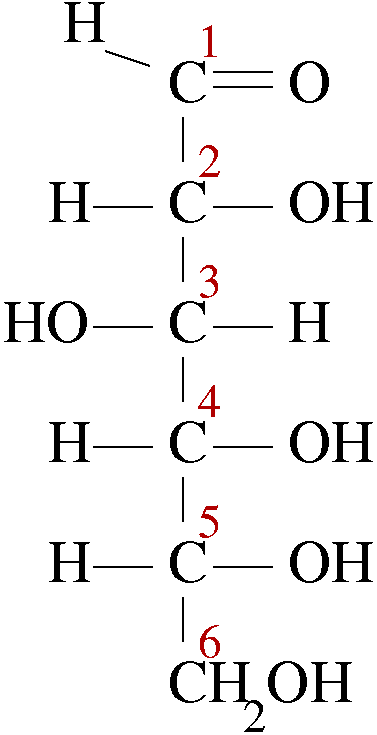
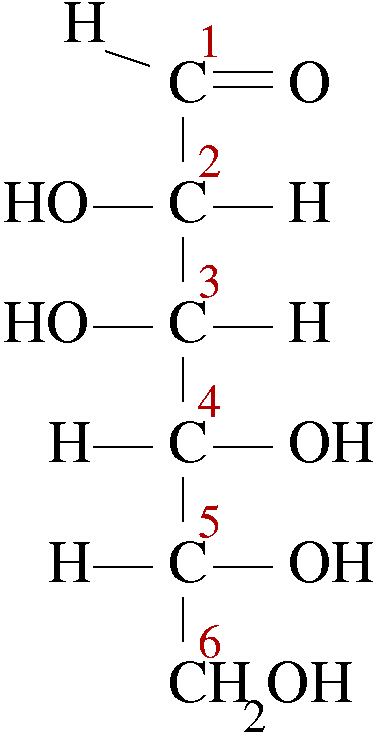
D-Glucose D-Mannose
The configuration of all chiral carbons from C2 to C5 is 2R, 3S, 4R and 5R. but its epimer D-Mannose has different arrangement at C2 and the configuration of all chiral carbons of D-Mannose becomes 2S, 3S, 4R and 5R. Therefore, we call them epimers as they differ only at C2 position, while the rest is identical. D-Maltose has two D-glucopyranose units, so it is entirely different from D-Glucose. Whereas D-Arabinose has a five carbon chain only.
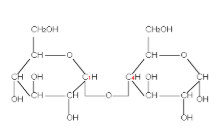
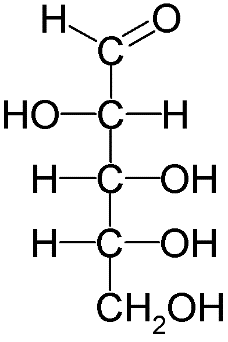
D-Maltose D-Arabinose
Hence, the correct option is (B).
Note:
Epimers are not mirror images and are non-superimposable to each other, so they can be called diastereomers as well. The configuration at chiral carbon can be identified by assigning priorities to the groups attached to the chiral carbon and check whether it is S or R configuration.
Complete step by step answer:
Epimers are the diastereomers that contain one or more chiral carbon but differ in their configuration at only one chiral carbon in their structures. Let us look at the structure of D-Glucose, the carbon chain is assigned numbers as per functional group priority. At its C2 position, the constituents are exchanged from right to left to left to right. This gives a new compound called D-Mannose.


D-Glucose D-Mannose
The configuration of all chiral carbons from C2 to C5 is 2R, 3S, 4R and 5R. but its epimer D-Mannose has different arrangement at C2 and the configuration of all chiral carbons of D-Mannose becomes 2S, 3S, 4R and 5R. Therefore, we call them epimers as they differ only at C2 position, while the rest is identical. D-Maltose has two D-glucopyranose units, so it is entirely different from D-Glucose. Whereas D-Arabinose has a five carbon chain only.


D-Maltose D-Arabinose
Hence, the correct option is (B).
Note:
Epimers are not mirror images and are non-superimposable to each other, so they can be called diastereomers as well. The configuration at chiral carbon can be identified by assigning priorities to the groups attached to the chiral carbon and check whether it is S or R configuration.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




