
Which of the following products cannot be obtained in ozonolysis of o-xylene?
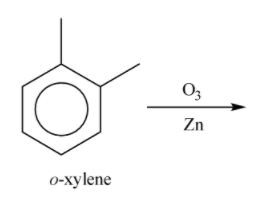
(A)-
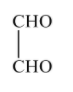
(B)-
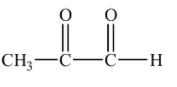
(C)-
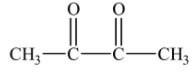
(D)-





Answer
574.2k+ views
Hint: Ozonolysis involves the addition of ozone to alkene, alkyne and aromatic hydrocarbons to form alkene ozonide, alkyne ozonide and triozonide, respectively. Cleavage of ozonide by Zn-${{H}_{2}}O$ gives carbonyl compounds, i.e. aldehydes and ketones.
Complete answer:
In the ozonolysis reaction of o-xylene, o-xylene forms xylene triozonide with ozone (${{O}_{3}}$) which upon decomposition with Zn-${{H}_{2}}O$ gives the following products:

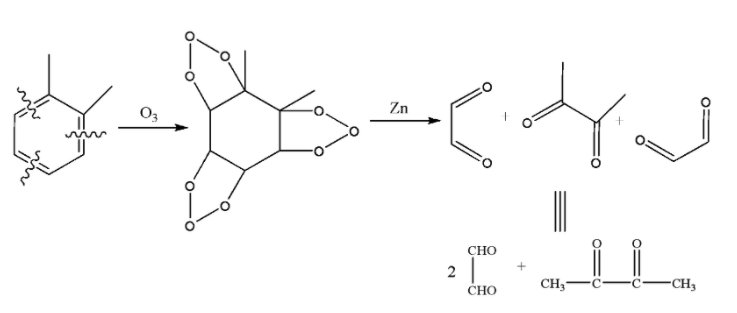
So, we can see that the products formed by the ozonolysis of o-xylene are
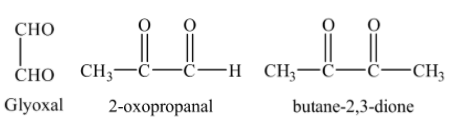
Now let us examine all the given options and find out the compound which is not formed during ozonolysis of xylene.
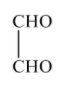
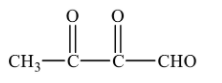
Glyoxal is formed during the ozonolysis. So, it cannot be the correct option to the given question.
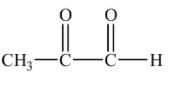
2-oxopropanal is also formed as one of the products in ozonolysis of o-xylene.

Ozonolysis of o-xylene also forms this diketone as one of the products. Thus, it is not the right option.
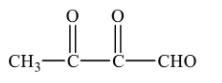
Since, 2,3-dioxobutanal is not formed in the ozonolysis of o-xylene, therefore, the correct option will be (D).
Additional information:
Ozonolysis reaction is useful in detecting the presence and position of double bonds in unsaturated compounds. Zinc in ozonolysis acts as a reducing agent. Ozonolysis of benzene forms glyoxal as the only product.
Note:
It is to be noted that benzene shows resonance. o-xylene is a derivative of benzene. The $\pi $-electrons in xylene are not fixed but are delocalized in the entire ring. So, carefully consider both the resonance structures of xylene and then deduce all the possible products that can be formed on ozonolysis.
Complete answer:
In the ozonolysis reaction of o-xylene, o-xylene forms xylene triozonide with ozone (${{O}_{3}}$) which upon decomposition with Zn-${{H}_{2}}O$ gives the following products:


So, we can see that the products formed by the ozonolysis of o-xylene are

Now let us examine all the given options and find out the compound which is not formed during ozonolysis of xylene.

Glyoxal is formed during the ozonolysis. So, it cannot be the correct option to the given question.

2-oxopropanal is also formed as one of the products in ozonolysis of o-xylene.

Ozonolysis of o-xylene also forms this diketone as one of the products. Thus, it is not the right option.

Since, 2,3-dioxobutanal is not formed in the ozonolysis of o-xylene, therefore, the correct option will be (D).
Additional information:
Ozonolysis reaction is useful in detecting the presence and position of double bonds in unsaturated compounds. Zinc in ozonolysis acts as a reducing agent. Ozonolysis of benzene forms glyoxal as the only product.
Note:
It is to be noted that benzene shows resonance. o-xylene is a derivative of benzene. The $\pi $-electrons in xylene are not fixed but are delocalized in the entire ring. So, carefully consider both the resonance structures of xylene and then deduce all the possible products that can be formed on ozonolysis.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




