
Which of the following reacts with phenol to give salicylaldehyde after hydrolysis?
A. Dichloro methane
B. Trichloromethane
C. Methyl chloride
D. None of the above
Answer
563.7k+ views
Hint:Phenol is an aromatic alcohol which is acidic in nature. The salicylaldehyde compound is an organic compound which has a formal group attached to the ortho position of the hydroxyl group of phenol.
Complete step by step answer: The reaction is an organic reaction known as Reimer–Tiemann reaction. It was given by the chemists Karl Reimer and Ferdinand Tiemann. The reaction is used for the ortho formylation of phenols.
The reaction is achieved by treating phenol with\[CHC{l_3}\] (chloroform) or trichloromethane in the presence of alkali hydroxide like \[NaOH\] (sodium hydroxide). The product of the reaction contains an aldehyde group (\[CHO\]) at the ortho position with respect to the hydroxyl group of benzene ring. The obtained product is named as o-hydroxy benzaldehyde.
Hence option B is the correct answer, i.e. trichloro methane.
The mechanism of the reaction begins with deprotonation of chloroform in presence of strong base forming the chloroform carbanion. An alpha-elimination occurs to give dichlorocarbene which is the reactive species. The phenol compound undergoes deprotonation generation of the phenoxide ion. The negative charge of the phenoxide ion is delocalized with the benzene ring, so this makes it more nucleophilic.
The nucleophilic benzene ring attacks the electron deficient dichlorocarbene producing the intermediate dichloromethyl substituted phenol which on hydrolysis leads to the formation of desired salicylaldehyde product. The mechanism is shown as
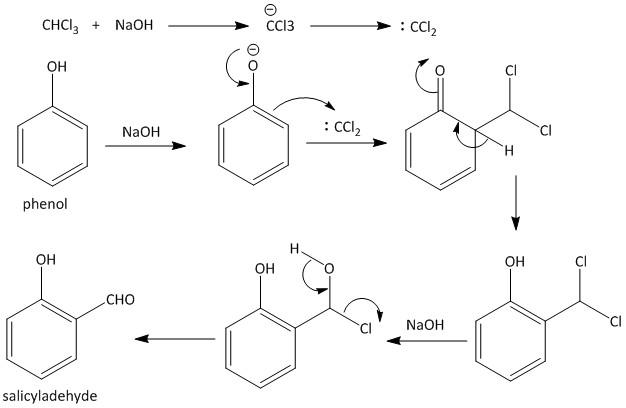
Hence option B is the correct answer.
Note:
Alkali metal hydroxides are not soluble in chloroform so the reaction is carried out using a biphasic solvent system. This biphasic solvent system contains phase-transfer catalysts or emulsifying agents which bring hydroxide solution and chloroform in the same phase for reaction to occur.
Complete step by step answer: The reaction is an organic reaction known as Reimer–Tiemann reaction. It was given by the chemists Karl Reimer and Ferdinand Tiemann. The reaction is used for the ortho formylation of phenols.
The reaction is achieved by treating phenol with\[CHC{l_3}\] (chloroform) or trichloromethane in the presence of alkali hydroxide like \[NaOH\] (sodium hydroxide). The product of the reaction contains an aldehyde group (\[CHO\]) at the ortho position with respect to the hydroxyl group of benzene ring. The obtained product is named as o-hydroxy benzaldehyde.
Hence option B is the correct answer, i.e. trichloro methane.
The mechanism of the reaction begins with deprotonation of chloroform in presence of strong base forming the chloroform carbanion. An alpha-elimination occurs to give dichlorocarbene which is the reactive species. The phenol compound undergoes deprotonation generation of the phenoxide ion. The negative charge of the phenoxide ion is delocalized with the benzene ring, so this makes it more nucleophilic.
The nucleophilic benzene ring attacks the electron deficient dichlorocarbene producing the intermediate dichloromethyl substituted phenol which on hydrolysis leads to the formation of desired salicylaldehyde product. The mechanism is shown as

Hence option B is the correct answer.
Note:
Alkali metal hydroxides are not soluble in chloroform so the reaction is carried out using a biphasic solvent system. This biphasic solvent system contains phase-transfer catalysts or emulsifying agents which bring hydroxide solution and chloroform in the same phase for reaction to occur.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




