
Which of the following structures represents vinyl carbinol?
(A) $ OH-C{{H}_{2}}-CH=C{{H}_{2}} $
(B)
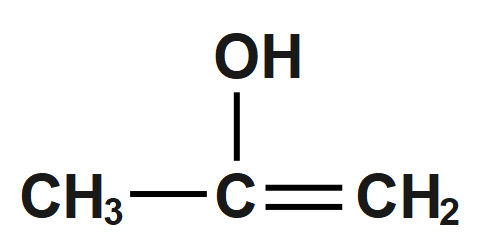
(C) $ C{{H}_{3}}-CH=CH-OH $
(D) $ C{{H}_{3}}-C(C{{H}_{2}}OH)=C{{H}_{2}} $

Answer
537.3k+ views
Hint: Initially you must be sure that the compound named vinyl carbinol is an alcohol. There is a simple method to figure out the correct option. Just check whether the group has branches or not and you will get your answers easily.
Complete step by step solution:
The structure of a vinyl group is equivalent to the molecular group when one hydrogen atom is removed from an ethene molecule. Therefore, it is also known as an ethenyl group. It contains two $ s{{p}^{2}} $ hybridized carbon atoms and three hydrogen atoms. The removed hydrogen atom can be replaced by any group of molecules, and it is denoted as $ R. $ Now for the case of alkyl group When one hydrogen atom is removed from the third carbon atom of a propane molecule, it is equivalent to an alkyl group. It contains two $ s{{p}^{2}} $ hybridized carbon atoms and one $ s{{p}^{3}} $ hybridized carbon atom. In other words, it is a methylene bridge $ \left( -C{{H}_{2}}- \right) $ attached to a vinyl group $ \left( -CH=C{{H}_{2}} \right). $
Therefore, correct answer is option A i.e. $ OH-C{{H}_{2}}-CH=C{{H}_{2}} $ .
Note:
Vinyl carbinol also known as $ 2- $ propanol. It is a colorless liquid. It has pungent odor which is used in making resins. Its plasticizers are highly irritating to mucous membranes and readily absorbed, causing depression and coma. It boils at $ 96.9{}^\circ C $ and its density is $ 852\text{ }kg/{{m}^{3}}. $ It mixes in any proportion with water, alcohol, or ether. Which means it is easily soluble in all the mediums. Vinyl alcohol is obtained by the hydrolysis of alkyl chloride. It is a kind of primary carbinol.
Complete step by step solution:
The structure of a vinyl group is equivalent to the molecular group when one hydrogen atom is removed from an ethene molecule. Therefore, it is also known as an ethenyl group. It contains two $ s{{p}^{2}} $ hybridized carbon atoms and three hydrogen atoms. The removed hydrogen atom can be replaced by any group of molecules, and it is denoted as $ R. $ Now for the case of alkyl group When one hydrogen atom is removed from the third carbon atom of a propane molecule, it is equivalent to an alkyl group. It contains two $ s{{p}^{2}} $ hybridized carbon atoms and one $ s{{p}^{3}} $ hybridized carbon atom. In other words, it is a methylene bridge $ \left( -C{{H}_{2}}- \right) $ attached to a vinyl group $ \left( -CH=C{{H}_{2}} \right). $
Therefore, correct answer is option A i.e. $ OH-C{{H}_{2}}-CH=C{{H}_{2}} $ .
Note:
Vinyl carbinol also known as $ 2- $ propanol. It is a colorless liquid. It has pungent odor which is used in making resins. Its plasticizers are highly irritating to mucous membranes and readily absorbed, causing depression and coma. It boils at $ 96.9{}^\circ C $ and its density is $ 852\text{ }kg/{{m}^{3}}. $ It mixes in any proportion with water, alcohol, or ether. Which means it is easily soluble in all the mediums. Vinyl alcohol is obtained by the hydrolysis of alkyl chloride. It is a kind of primary carbinol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




