
Which of the following will give a pleasant smell of ester when heated with ethanol and a small quantity of sulphuric acid?
A. $C{H_2}C{H_2}OH$
B. $C{H_3}OH$
C. $C{H_3}COOH$
D. $C{H_3}CHO$
Answer
582.3k+ views
Hint: Ester is an organic compound with two hydrocarbon chains separated by oxygen and a double bonded oxygen on the carbon adjacent to the oxygen separating the two hydrocarbon groups. Esters are used as perfumes, flavorings and also as solvents.
Step by step answer: The chemical formula of ethanol is $C{H_3}C{H_2}OH$
(i) $C{H_2}C{H_2}OH$ is 2-Hydroxyethyl radical. It does not react with ethanol. So there is no formation of any ester.
(ii) $C{H_3}OH$ is also known as methanol. Methanol and Ethanol both are alcohols, as they are solutions they mix readily. Ethanol is the only drinkable form of alcohol and Methanol is a non-drinking type of alcohol which is also known as methyl alcohol. Methanol is a very poisonous alcohol. When they both react there can be a possibility of formation of ethers but not esters. So when methanol and ethanol reacts there is no formation of an ester.
(iii) $C{H_3}COOH$ is known as acetic acid and it is also known as Ethanoic acid. Ethanoic acid reacts with ethanol in the presence of concentrated sulphuric acid, which acts as a catalyst, to produce an ester called ethyl ethanoate. The reaction is very slow and a reversible reaction. The process of formation of esters from carboxylic acids and alcohols in the presence of sulphuric acid acting as a catalyst is known as esterification.
$C{H_3}COOH + C{H_3}C{H_2}0H\overset {Conc.{H_2}S{O_4}} \longleftrightarrow C{H_3}COOC{H_2}C{H_3} + {H_2}O$
(iv) $C{H_3}CHO$ is known as acetaldehyde and also known as Ethanal. Ethanal is a product after oxidizing ethanol. Ethanal and ethanol only react with each other in the presence of acidic medium. These two react to form acetal.
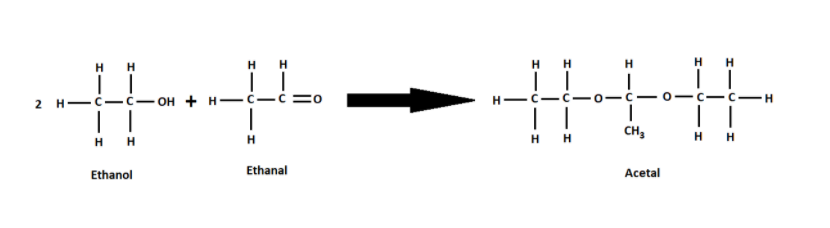
Therefore, only acetic acid when reacted with ethanol in the presence of concentrated sulphuric acid as a catalyst produces an ester which gives a pleasant smell.
The correct option is Option C, $C{H_3}COOH$.
Note: Catalyst is a compound or a substance that increases the rate of a chemical reaction without itself undergoing any chemical changes. Ether is an organic compound with two hydrocarbon chains separated by oxygen. Ether and ester are the same with little difference. Do not confuse an ester with ether.
Step by step answer: The chemical formula of ethanol is $C{H_3}C{H_2}OH$
(i) $C{H_2}C{H_2}OH$ is 2-Hydroxyethyl radical. It does not react with ethanol. So there is no formation of any ester.
(ii) $C{H_3}OH$ is also known as methanol. Methanol and Ethanol both are alcohols, as they are solutions they mix readily. Ethanol is the only drinkable form of alcohol and Methanol is a non-drinking type of alcohol which is also known as methyl alcohol. Methanol is a very poisonous alcohol. When they both react there can be a possibility of formation of ethers but not esters. So when methanol and ethanol reacts there is no formation of an ester.
(iii) $C{H_3}COOH$ is known as acetic acid and it is also known as Ethanoic acid. Ethanoic acid reacts with ethanol in the presence of concentrated sulphuric acid, which acts as a catalyst, to produce an ester called ethyl ethanoate. The reaction is very slow and a reversible reaction. The process of formation of esters from carboxylic acids and alcohols in the presence of sulphuric acid acting as a catalyst is known as esterification.
$C{H_3}COOH + C{H_3}C{H_2}0H\overset {Conc.{H_2}S{O_4}} \longleftrightarrow C{H_3}COOC{H_2}C{H_3} + {H_2}O$
(iv) $C{H_3}CHO$ is known as acetaldehyde and also known as Ethanal. Ethanal is a product after oxidizing ethanol. Ethanal and ethanol only react with each other in the presence of acidic medium. These two react to form acetal.

Therefore, only acetic acid when reacted with ethanol in the presence of concentrated sulphuric acid as a catalyst produces an ester which gives a pleasant smell.
The correct option is Option C, $C{H_3}COOH$.
Note: Catalyst is a compound or a substance that increases the rate of a chemical reaction without itself undergoing any chemical changes. Ether is an organic compound with two hydrocarbon chains separated by oxygen. Ether and ester are the same with little difference. Do not confuse an ester with ether.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




