
Which of these configurations are achiral:
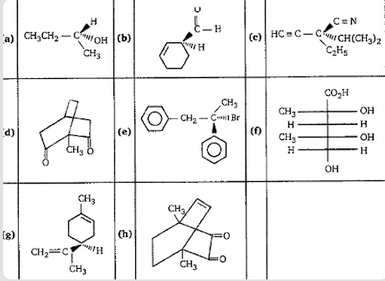
A. e, h
B. a, e
C. d, h
D. none of these.

Answer
593.1k+ views
Hint: Those molecules which are similar and cannot superimpose on the mirror image are called chiral molecules whereas those molecules which can superimpose on their mirror image are known as achiral molecules.
Complete answer:
-In the given figures, we have to identify the achiral molecules.
-As we know that achiral molecules are that configuration in which the mirror images are superimposable on each other i.e. they can completely overlap on each other.
-Achiral molecules also have a plane of symmetry which divides them into equal parts.
-Among all the configurations, only d and h configurations are considered as the achiral molecule because molecule d and h consist of symmetry.
-The plane of symmetrical is not found in the chiral molecules because they cannot be divided into two halves.
-Due to which chiral molecules are optically active and achiral molecules are optically inactive.
-Moreover, the presence of bisecting planes in a molecule that separates the molecule into two equal parts also confirms that it is an achiral molecule.
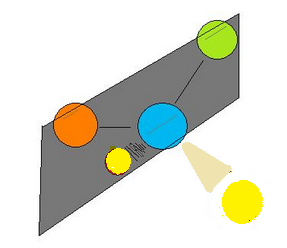
-The above figures show the bisecting plane of a molecule.
-That’s why d and h are the achiral molecules.
Therefore, option C is the correct answer.
Note: Chiral and achiral molecules are opposite of each other in the structure as well as in properties. Whereas the enantiomer and chiral molecules have the same physical and chemical properties. Enantiomers are the pair of molecules which are a mirror image of each other.
Complete answer:
-In the given figures, we have to identify the achiral molecules.
-As we know that achiral molecules are that configuration in which the mirror images are superimposable on each other i.e. they can completely overlap on each other.
-Achiral molecules also have a plane of symmetry which divides them into equal parts.
-Among all the configurations, only d and h configurations are considered as the achiral molecule because molecule d and h consist of symmetry.
-The plane of symmetrical is not found in the chiral molecules because they cannot be divided into two halves.
-Due to which chiral molecules are optically active and achiral molecules are optically inactive.
-Moreover, the presence of bisecting planes in a molecule that separates the molecule into two equal parts also confirms that it is an achiral molecule.

-The above figures show the bisecting plane of a molecule.
-That’s why d and h are the achiral molecules.
Therefore, option C is the correct answer.
Note: Chiral and achiral molecules are opposite of each other in the structure as well as in properties. Whereas the enantiomer and chiral molecules have the same physical and chemical properties. Enantiomers are the pair of molecules which are a mirror image of each other.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




