
Which one of the following is aromatic?
(A) Cyclopentadienyl cation
(B) Cyclooctatetraene
(C) Cycloheptatriene
(D) Cycloheptatrienyl cation
Answer
528.4k+ views
Hint: A compound is said to be aromatic if it has (4n+2)π electrons which are delocalised in the structure and the structure is planar. This is Hückel's rule. Also it should be cyclic, planar and all π-electrons should be delocalised.
Complete step by step answer:
-First we should start from the aromaticity of all 4 structures. To be known as an aromatic compound any structure should fulfil the following conditions:
-The molecule should be cyclic
- These π electrons should be delocalised in the ring or structure
-The structure should be planar
-It should follow Hückel’s rule or the (4n+2) rule.
-According to the Hückel’s rule (also known as (4n+2) rule) any compound having number of π electrons equal to (4n+2) where n is 0 or any other whole natural number (1, 2, 3 ….) is said to be an aromatic compound.
-Now let us see all the options one by one to check whether they are aromatic or not.
(A) Cyclopentadienyl cation (${C_5}{H_5}^ + $): Its structure is:
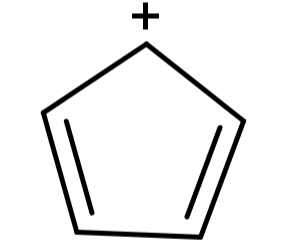
Here we can see that the structure is cyclic, planar and the electrons can be delocalised as well but there are 4π electrons which do not satisfy the Hückel’s rule. So, this compound is not aromatic.
(B) Cyclooctatetraene (${C_8}{H_8}$): Its structure is:
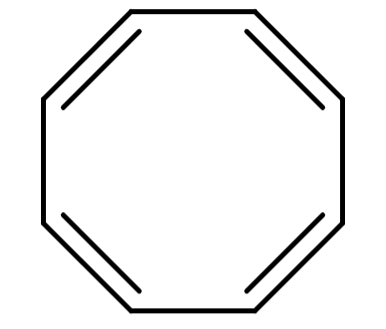
The structure of Cyclooctatetraene is cyclic but it is non-planar. Also it has 8π electrons which do not satisfy Hückel's rule. So, this compound is also not aromatic.
(C) Cycloheptatriene (${C_7}{H_8}$): Its structure is:
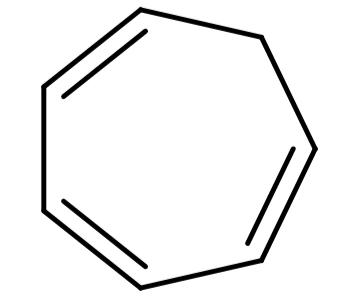
This structure is cyclic, planar and has 6π electrons so follows Hückel’s rule also, but all the π-electrons are not in conjugation to each other. So, this compound is also not aromatic.
(D) Cycloheptatrienyl cation (${C_7}{H_7}^ + $): Its structure is:
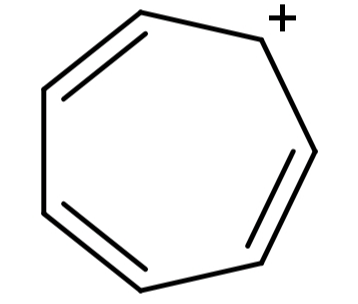
This structure is cyclic, planar, the presence of positive charge causes full delocalisation of the π-electrons and it has 6π electrons, so it follows Hückel’s rule also. Since it satisfies all the conditions for any compound to be aromatic, it is an aromatic compound.
So, the correct answer is “Option D”.
Note: Major mistake that we can make here is while finding a compound’s aromaticity. Always make sure you declare any compound aromatic only if it fulfils all required conditions. Even if it fails to fulfil one single condition it cannot be called aromatic.
Also if any structure is planar, cyclic and conjugated but has 4n π-electrons, then it will be anti aromatic. But if the number of π-electrons are neither (4n+2) nor (4n) then it will be a non-aromatic compound.
Complete step by step answer:
-First we should start from the aromaticity of all 4 structures. To be known as an aromatic compound any structure should fulfil the following conditions:
-The molecule should be cyclic
- These π electrons should be delocalised in the ring or structure
-The structure should be planar
-It should follow Hückel’s rule or the (4n+2) rule.
-According to the Hückel’s rule (also known as (4n+2) rule) any compound having number of π electrons equal to (4n+2) where n is 0 or any other whole natural number (1, 2, 3 ….) is said to be an aromatic compound.
-Now let us see all the options one by one to check whether they are aromatic or not.
(A) Cyclopentadienyl cation (${C_5}{H_5}^ + $): Its structure is:

Here we can see that the structure is cyclic, planar and the electrons can be delocalised as well but there are 4π electrons which do not satisfy the Hückel’s rule. So, this compound is not aromatic.
(B) Cyclooctatetraene (${C_8}{H_8}$): Its structure is:

The structure of Cyclooctatetraene is cyclic but it is non-planar. Also it has 8π electrons which do not satisfy Hückel's rule. So, this compound is also not aromatic.
(C) Cycloheptatriene (${C_7}{H_8}$): Its structure is:

This structure is cyclic, planar and has 6π electrons so follows Hückel’s rule also, but all the π-electrons are not in conjugation to each other. So, this compound is also not aromatic.
(D) Cycloheptatrienyl cation (${C_7}{H_7}^ + $): Its structure is:

This structure is cyclic, planar, the presence of positive charge causes full delocalisation of the π-electrons and it has 6π electrons, so it follows Hückel’s rule also. Since it satisfies all the conditions for any compound to be aromatic, it is an aromatic compound.
So, the correct answer is “Option D”.
Note: Major mistake that we can make here is while finding a compound’s aromaticity. Always make sure you declare any compound aromatic only if it fulfils all required conditions. Even if it fails to fulfil one single condition it cannot be called aromatic.
Also if any structure is planar, cyclic and conjugated but has 4n π-electrons, then it will be anti aromatic. But if the number of π-electrons are neither (4n+2) nor (4n) then it will be a non-aromatic compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




