
Which property of white phosphorous is common to red phosphorous:
A.It burns when heated in air
B.It reacts with hot caustic soda solution to give phosphine
C.It shows chemiluminescence
D.It is soluble in carbon disulphide
Answer
579.9k+ views
Hint: Allotropy can be basically explained as the property exhibited by certain substances that enables them to exist in their elemental state in two or more physical forms. These physical forms may differ in occurrence of the molecules that might contain different numbers of atoms or maybe there might be change in the arrangements of atoms in crystalline solids.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Phosphorus exists in many allotropic forms. But two of the most prevalent allotropes of phosphorus are white and red solids. These solids are known as white phosphorus and red phosphorus respectively. Some of the physical and chemical properties of these allotropes and be explained as follows:
1. White phosphorous:
a)The molecular formula of white phosphorous is \[{P_4}\] with the structure:
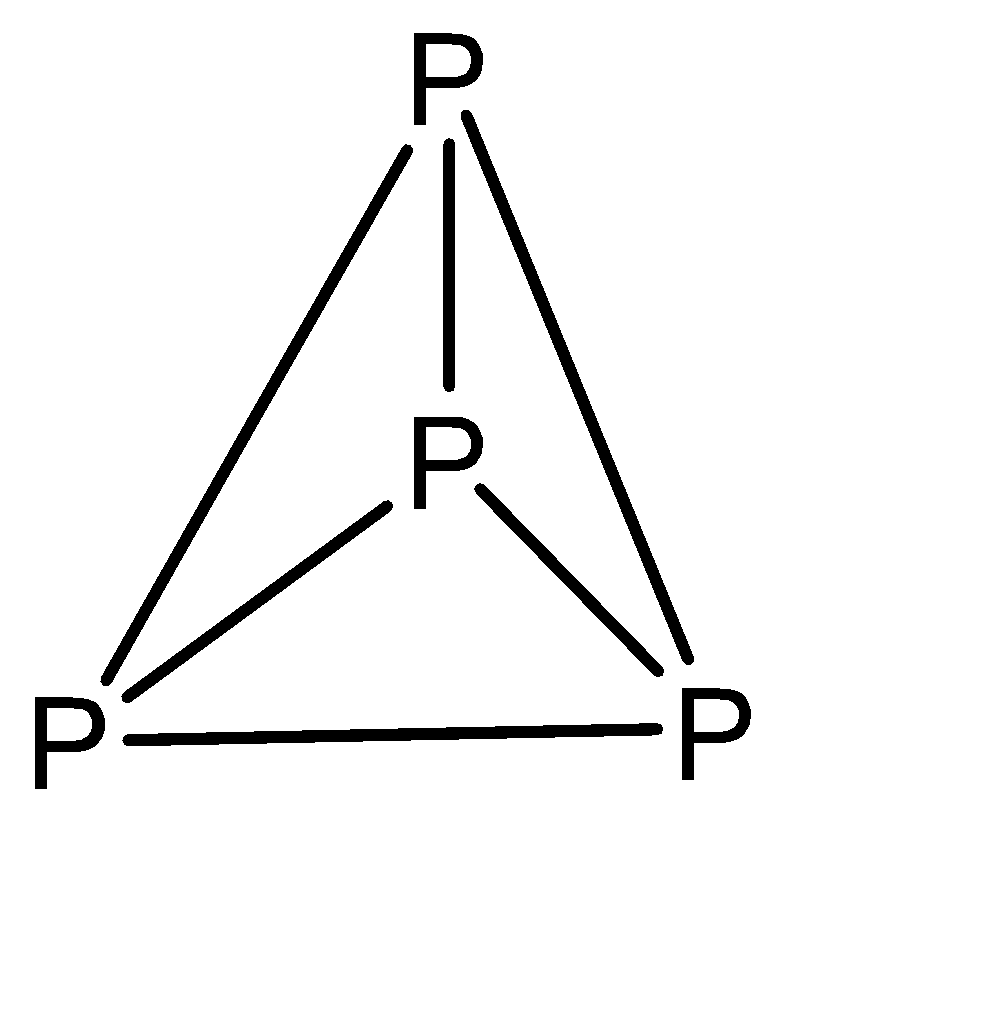
b)White phosphorus ignites at about 303 K in air. This ignition temperature may increase when the air is dry.
c)White phosphorus reacts with hot caustic soda solution to give phosphine and sodium hypophosphite.
d)White phosphorus shows chemiluminescence
e)white phosphorus is soluble in carbon disulphide
2.Red Phosphorous:
a)The molecular formula of red phosphorus is \[{P_4}\] with the structure:
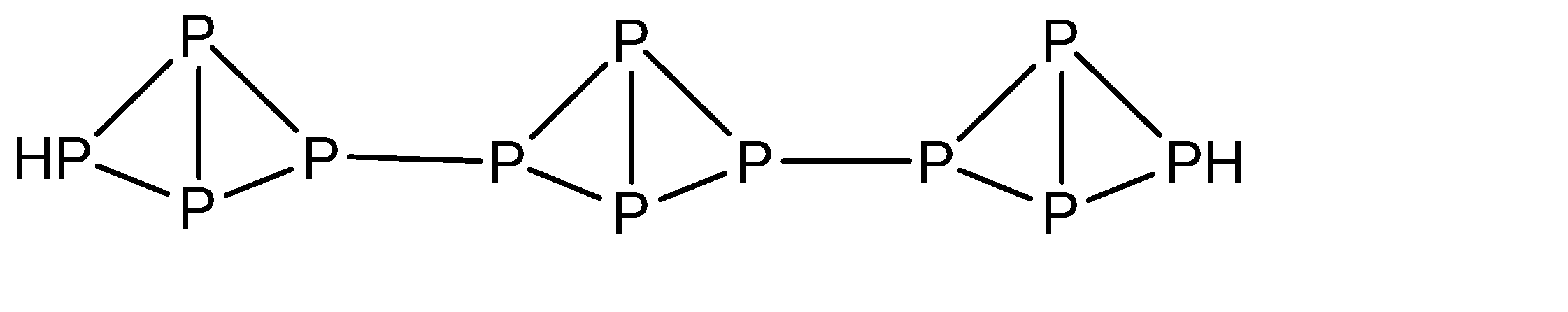
b)Red phosphorus burns when heated in air
c)Red phosphorus does not react with hot caustic soda solution to give phosphine
d)It does not show chemiluminescence
e)It is insoluble in carbon disulphide
Hence, Option A is the correct option
Note: Solid violet and black allotropes also are known. Black phosphorus is an allotrope of phosphorus consisting of multiple layers with two-dimensional structures, weakly bonded to one another by van der Waals forces. Elemental phosphorus is also known to exist in the gaseous form.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Phosphorus exists in many allotropic forms. But two of the most prevalent allotropes of phosphorus are white and red solids. These solids are known as white phosphorus and red phosphorus respectively. Some of the physical and chemical properties of these allotropes and be explained as follows:
1. White phosphorous:
a)The molecular formula of white phosphorous is \[{P_4}\] with the structure:

b)White phosphorus ignites at about 303 K in air. This ignition temperature may increase when the air is dry.
c)White phosphorus reacts with hot caustic soda solution to give phosphine and sodium hypophosphite.
d)White phosphorus shows chemiluminescence
e)white phosphorus is soluble in carbon disulphide
2.Red Phosphorous:
a)The molecular formula of red phosphorus is \[{P_4}\] with the structure:

b)Red phosphorus burns when heated in air
c)Red phosphorus does not react with hot caustic soda solution to give phosphine
d)It does not show chemiluminescence
e)It is insoluble in carbon disulphide
Hence, Option A is the correct option
Note: Solid violet and black allotropes also are known. Black phosphorus is an allotrope of phosphorus consisting of multiple layers with two-dimensional structures, weakly bonded to one another by van der Waals forces. Elemental phosphorus is also known to exist in the gaseous form.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




