
Which type of intermolecular forces exist between
A.\[{H_2}S\] molecules
B.\[C{l_2}\] molecules
C.\[CC{l_4}\] molecules
Answer
514.8k+ views
Hint: Intermolecular forces are forces that exist between molecules. These are the forces which mediate interaction between molecules, including forces of attraction or repulsion which act between atoms and other types of neighboring particles, for example, atoms or ions.
Types of intermolecular forces of attraction are:
-Dipole-Dipole interactions
-Ion-Dipole interactions
-Ion- Induced dipole interactions
-Dipole-Induced dipole interactions
-London forces / Dispersion forces
Complete answer: The Lewis structure of \[{H_2}S\] is:
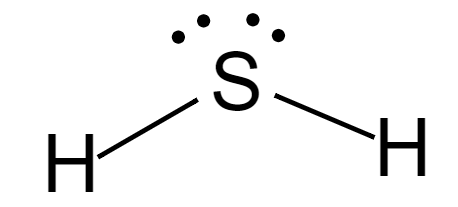
Here, the central atom is sulphur and it has 4 entities around it, which are two hydrogen atoms and two lone pairs. According to VSEPR theory, the shape of the molecule is bent and because of the asymmetrical shape, the molecule is polar.
The intermolecular force that exists between polar molecules is dipole-dipole forces.
Hence, Dipole-Dipole forces exist between \[{H_2}S\] molecules.
A.\[C{l_2}\] molecules
The Lewis structure of \[C{l_2}\] is:

Here, there is no central atom, and both atoms are the same, that is \[Cl\]. From VSEPR theory, this can be concluded that the molecular shape is linear, and because of the symmetrical shape, the molecule is nonpolar.
Nonpolar molecules lack permanent dipoles and hence cannot take part in dipole-dipole interaction, and there are no other characteristics that give this molecule the ability to have stronger intermolecular forces.
So, the intermolecular force that exists between \[C{l_2}\] molecules is London forces or Dispersion forces.
C.\[CC{l_4}\] molecules
The Lewis dot structure of \[CC{l_4}\] is:
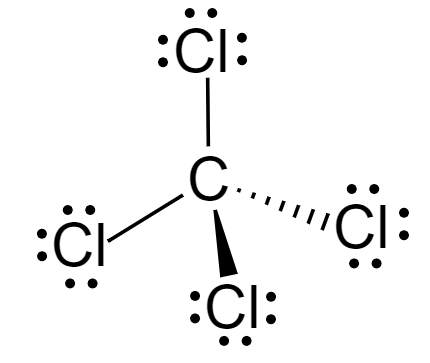
Here, the central atom is carbon and has four \[Cl\] atoms around it, and no lone pairs. According to VSEPR theory, it can be concluded that the molecule is tetrahedral in shape, and therefore it is a symmetrical molecule. This means that the molecule is overall nonpolar.
Hence, the intermolecular force that exists between \[CC{l_4}\] molecules is London forces or Dispersion forces.
ADDITIONAL INFORMATION: Various physical and chemical properties of a substance are dependent on the intermolecular forces. For example: the boiling point of a substance is proportional to the strength of its intermolecular forces. The stronger the intermolecular forces, the higher the boiling point.
A hydrogen bond is an intermolecular force that forms a special type of dipole-dipole attraction when a hydrogen atom bonded to a strongly electronegative atom exists in the vicinity of another electronegative atom with a lone pair of electrons.
Note:
Vander Waals dispersion forces are stronger in molecules which are not compact, but possess long chains of elements. The reason is that it is more convenient and easier to displace the electrons as the forces of attraction between electrons and the protons in the nucleus are weaker.
Types of intermolecular forces of attraction are:
-Dipole-Dipole interactions
-Ion-Dipole interactions
-Ion- Induced dipole interactions
-Dipole-Induced dipole interactions
-London forces / Dispersion forces
Complete answer: The Lewis structure of \[{H_2}S\] is:

Here, the central atom is sulphur and it has 4 entities around it, which are two hydrogen atoms and two lone pairs. According to VSEPR theory, the shape of the molecule is bent and because of the asymmetrical shape, the molecule is polar.
The intermolecular force that exists between polar molecules is dipole-dipole forces.
Hence, Dipole-Dipole forces exist between \[{H_2}S\] molecules.
A.\[C{l_2}\] molecules
The Lewis structure of \[C{l_2}\] is:

Here, there is no central atom, and both atoms are the same, that is \[Cl\]. From VSEPR theory, this can be concluded that the molecular shape is linear, and because of the symmetrical shape, the molecule is nonpolar.
Nonpolar molecules lack permanent dipoles and hence cannot take part in dipole-dipole interaction, and there are no other characteristics that give this molecule the ability to have stronger intermolecular forces.
So, the intermolecular force that exists between \[C{l_2}\] molecules is London forces or Dispersion forces.
C.\[CC{l_4}\] molecules
The Lewis dot structure of \[CC{l_4}\] is:

Here, the central atom is carbon and has four \[Cl\] atoms around it, and no lone pairs. According to VSEPR theory, it can be concluded that the molecule is tetrahedral in shape, and therefore it is a symmetrical molecule. This means that the molecule is overall nonpolar.
Hence, the intermolecular force that exists between \[CC{l_4}\] molecules is London forces or Dispersion forces.
ADDITIONAL INFORMATION: Various physical and chemical properties of a substance are dependent on the intermolecular forces. For example: the boiling point of a substance is proportional to the strength of its intermolecular forces. The stronger the intermolecular forces, the higher the boiling point.
A hydrogen bond is an intermolecular force that forms a special type of dipole-dipole attraction when a hydrogen atom bonded to a strongly electronegative atom exists in the vicinity of another electronegative atom with a lone pair of electrons.
Note:
Vander Waals dispersion forces are stronger in molecules which are not compact, but possess long chains of elements. The reason is that it is more convenient and easier to displace the electrons as the forces of attraction between electrons and the protons in the nucleus are weaker.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




