
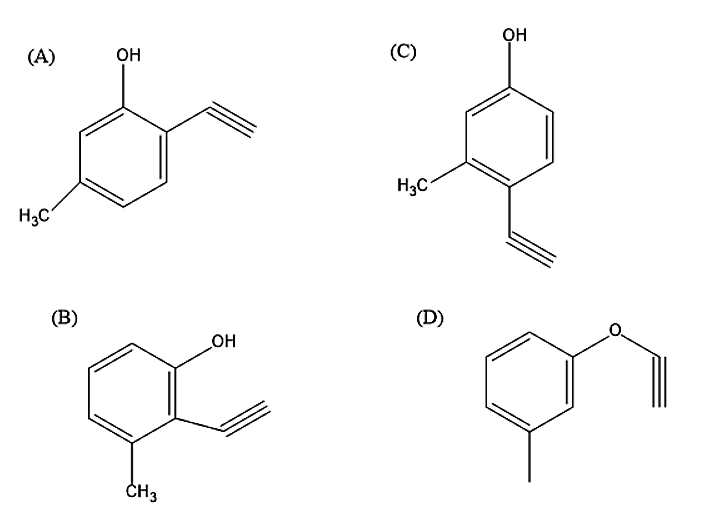
Which will be the major product when m-cresol is reacted with propargyl bromide \[(HC \equiv C - C{H_2}Br)\] in presence of \[{K_2}C{O_3}\] in acetone?

Answer
569.7k+ views
Hint: The reaction in which meta-cresol reacts with propargyl bromide in the presence of \[{K_2}C{O_3}\] in acetone is a nucleophilic bimolecular reaction which gives ethers. Such a reaction is known as Williamson’s synthesis. Here \[{K_2}C{O_3}\] will be a base which will abstract protons and acetone will be a solvent.
Complete answer:
-Williamson synthesis is an organic reaction in which ether is formed from an organohalide and deprotonated alcohol (alkoxide).
-The reaction typically involves the reaction of an alkoxide ion with a primary alkyl halide via a \[{S_N}2\] reaction which was developed by Alexander Williamson in 1850.
-The general reaction mechanism of Williamson’s synthesis is given as-
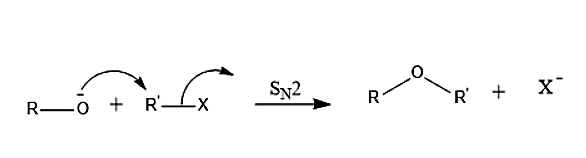
-Williamson ether reaction is a \[{S_N}2\] reaction in which a nucleophile attacks an electrophile from backside in a concerted mechanism. To occur a \[{S_N}2\] reaction there must be a good leaving group which is strongly electronegative, commonly a halide. The alkoxide ion acts as a nucleophile which attacks the electrophilic carbon which is mostly an alkyl tosylate or an alkyl halide. The leaving site here must be a primary carbon because secondary and tertiary leaving sites generally proceed as an elimination reaction rather than substitution reaction.
-Following the above mechanism, let us now react meta-cresol with propargyl bromide in the presence of \[{K_2}C{O_3}\] in acetone-
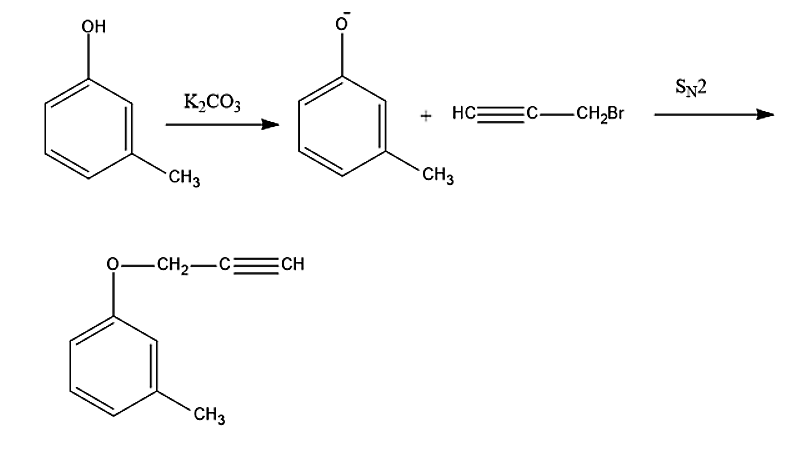
So, the correct answer is “Option D”.
Note: Let us now see the scopes of Williamson’s synthesis. Williamson’s synthesis is of broad scope in the industrial as well as laboratory preparation of ether. Both symmetrical and asymmetrical ethers can be easily prepared by this method. Despite this advantage of Williamson’s synthesis, it also has some limitations. Tertiary alkyl halides or sterically hindered primary or secondary alkyl halides tend to under elimination reactions rather than substitution reactions in the presence of alkoxide. Alkali phenoxides may undergo carbon-alkylation in addition to expected oxide-alkylation. This process of preparing ethers is too limited to be of any practical value for synthetic organic chemists.
Complete answer:
-Williamson synthesis is an organic reaction in which ether is formed from an organohalide and deprotonated alcohol (alkoxide).
-The reaction typically involves the reaction of an alkoxide ion with a primary alkyl halide via a \[{S_N}2\] reaction which was developed by Alexander Williamson in 1850.
-The general reaction mechanism of Williamson’s synthesis is given as-

-Williamson ether reaction is a \[{S_N}2\] reaction in which a nucleophile attacks an electrophile from backside in a concerted mechanism. To occur a \[{S_N}2\] reaction there must be a good leaving group which is strongly electronegative, commonly a halide. The alkoxide ion acts as a nucleophile which attacks the electrophilic carbon which is mostly an alkyl tosylate or an alkyl halide. The leaving site here must be a primary carbon because secondary and tertiary leaving sites generally proceed as an elimination reaction rather than substitution reaction.
-Following the above mechanism, let us now react meta-cresol with propargyl bromide in the presence of \[{K_2}C{O_3}\] in acetone-

So, the correct answer is “Option D”.
Note: Let us now see the scopes of Williamson’s synthesis. Williamson’s synthesis is of broad scope in the industrial as well as laboratory preparation of ether. Both symmetrical and asymmetrical ethers can be easily prepared by this method. Despite this advantage of Williamson’s synthesis, it also has some limitations. Tertiary alkyl halides or sterically hindered primary or secondary alkyl halides tend to under elimination reactions rather than substitution reactions in the presence of alkoxide. Alkali phenoxides may undergo carbon-alkylation in addition to expected oxide-alkylation. This process of preparing ethers is too limited to be of any practical value for synthetic organic chemists.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




