
Which xylene gives only one monobromo derivative?
Answer
517.2k+ views
Hint: Xylene is an organic compound whose molecular formula is \[{C_8}{H_{10}}\]. It is also called xylol or dimethylbenzene. It is an insoluble compound and is flammable in nature. It is found naturally in coal tar, petroleum and during forest fires.
Complete answer: There are three isomers of xylene, which are ortho-xylene, para-xylene and meta-xylene.
Bromination of ortho-xylene:
When bromination of ortho-xylene takes place, then the two sites are available for the attack of bromine atoms i.e., ortho and para position because the methyl group is ortho-para directing. Hence formation of two monobromo derivative products takes place. The reaction proceeds as follows:
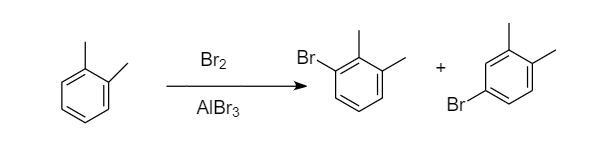
Bromination of para-xylene:
When bromination of para-xylene takes place, then only one site is available for the attack of bromine atoms i.e., ortho position because methyl group is ortho-para directing and para position is blocked by the methyl group. Hence formation of only one monobromo derivative product takes place. The reaction proceeds as follows:
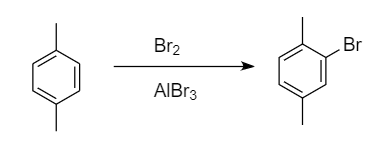
Bromination of meta-xylene:
When bromination of meta-xylene takes place, then there are three sites available for the attack of bromine atoms. As the methyl group is ortho-para directing, so there is one ortho position for each methyl group i.e., overall, two ortho positions and one para position is present. Hence formation of three monobromo derivative products takes place. The reaction proceeds as follows:
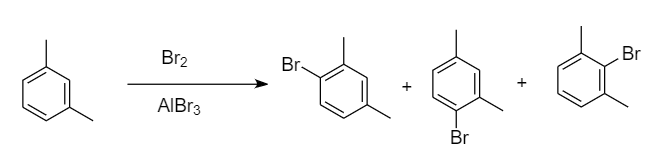
Therefore, the monobromo derivative is given by para-xylene only.
Note:
Para-xylene is majorly used as a building block (feedstock) to manufacture industrial chemicals like dimethyl terephthalic acid, purified terephthalic acid, etc. It is also used to form fabrics and home furnishing items like curtains, bedsheet, bedspreads, etc.
Complete answer: There are three isomers of xylene, which are ortho-xylene, para-xylene and meta-xylene.
Bromination of ortho-xylene:
When bromination of ortho-xylene takes place, then the two sites are available for the attack of bromine atoms i.e., ortho and para position because the methyl group is ortho-para directing. Hence formation of two monobromo derivative products takes place. The reaction proceeds as follows:

Bromination of para-xylene:
When bromination of para-xylene takes place, then only one site is available for the attack of bromine atoms i.e., ortho position because methyl group is ortho-para directing and para position is blocked by the methyl group. Hence formation of only one monobromo derivative product takes place. The reaction proceeds as follows:

Bromination of meta-xylene:
When bromination of meta-xylene takes place, then there are three sites available for the attack of bromine atoms. As the methyl group is ortho-para directing, so there is one ortho position for each methyl group i.e., overall, two ortho positions and one para position is present. Hence formation of three monobromo derivative products takes place. The reaction proceeds as follows:

Therefore, the monobromo derivative is given by para-xylene only.
Note:
Para-xylene is majorly used as a building block (feedstock) to manufacture industrial chemicals like dimethyl terephthalic acid, purified terephthalic acid, etc. It is also used to form fabrics and home furnishing items like curtains, bedsheet, bedspreads, etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




