
Write bond line formula for: ${\rm{Heptan - 4 - one}}$
Answer
583.2k+ views
Hint:
The bond line formula for a given compound can be written by using the bonding between the various atoms/groups present in the compound.
Complete step by step solution
In chemistry, we have different types of formulae that can be used to represent a given molecule. Let’s have a brief look at some of them:
- Complete structural formula: For writing a complete structural formula of a compound, dashes are used to represent all the bonds between the atoms. Single dash for single bond, double dash for double bond and so on.
- Condensed structural formula: While writing condensed structural formula for a compound, some or all dashes are removed. We write the number of identical atoms or groups attached to an atom by using subscript. We can also use parenthesis for groups at times.
- Bond-line formula: In this case, we use lines to represent carbon-carbon bonds. So, a carbon chain would be represented in a zig-zag manner. Carbon and hydrogen atoms are omitted and only hetero-atoms are shown.
For example, three different structural formula for propane can be written as follows:
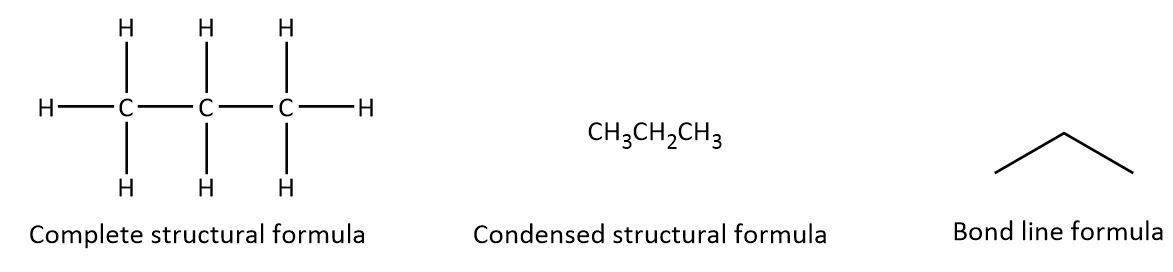
As we can see that in the complete structural formula of propane, all the atoms are shown along with single covalent bonds between them whereas in condensed formula first carbon attached to three hydrogen atoms, second one attached to two hydrogen atoms and the last one with three hydrogen atoms is written by using the atomic symbols and subscript only. In the bond line formula, all the atomic symbols have been omitted and we have a zig-zag line with three carbons.
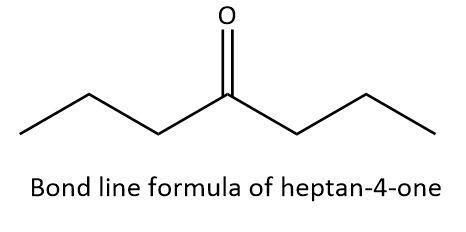
Now, let’s write the bond line formula for \[{\rm{heptan - 4 - one}}\]. In \[{\rm{heptan - 4 - one}}\], we have a carbon chain of seven atoms (hept) and we have a carbonyl group attached at the fourth carbon atoms. So, we will use a zig-zag line of seven carbon atoms, a double line at fourth carbon with an oxygen atom attached to it as follows:
Note:
We have to make sure that the carbonyl group is positioned correctly and the oxygen atom has been shown.
The bond line formula for a given compound can be written by using the bonding between the various atoms/groups present in the compound.
Complete step by step solution
In chemistry, we have different types of formulae that can be used to represent a given molecule. Let’s have a brief look at some of them:
- Complete structural formula: For writing a complete structural formula of a compound, dashes are used to represent all the bonds between the atoms. Single dash for single bond, double dash for double bond and so on.
- Condensed structural formula: While writing condensed structural formula for a compound, some or all dashes are removed. We write the number of identical atoms or groups attached to an atom by using subscript. We can also use parenthesis for groups at times.
- Bond-line formula: In this case, we use lines to represent carbon-carbon bonds. So, a carbon chain would be represented in a zig-zag manner. Carbon and hydrogen atoms are omitted and only hetero-atoms are shown.
For example, three different structural formula for propane can be written as follows:

As we can see that in the complete structural formula of propane, all the atoms are shown along with single covalent bonds between them whereas in condensed formula first carbon attached to three hydrogen atoms, second one attached to two hydrogen atoms and the last one with three hydrogen atoms is written by using the atomic symbols and subscript only. In the bond line formula, all the atomic symbols have been omitted and we have a zig-zag line with three carbons.
Now, let’s write the bond line formula for \[{\rm{heptan - 4 - one}}\]. In \[{\rm{heptan - 4 - one}}\], we have a carbon chain of seven atoms (hept) and we have a carbonyl group attached at the fourth carbon atoms. So, we will use a zig-zag line of seven carbon atoms, a double line at fourth carbon with an oxygen atom attached to it as follows:

Note:
We have to make sure that the carbonyl group is positioned correctly and the oxygen atom has been shown.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




