
Write chemical reactions to prepare the Nylon-6.
Answer
589.8k+ views
Hint: Nylon-6 is also known as Polycaprolactam which can be prepared from Caprolactam.
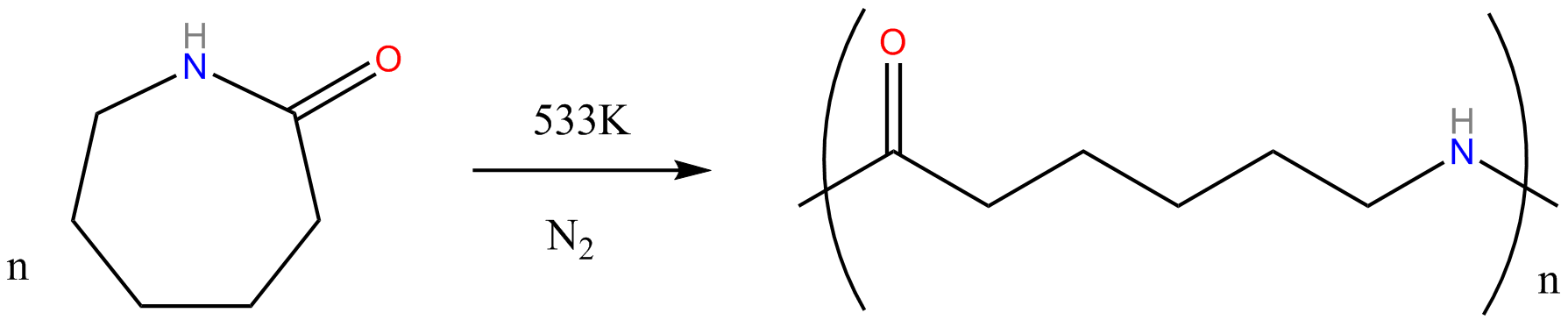
-Nylon-6 is synthesised by ring opening polymerisation of caprolactam. There are six carbon atoms in the Caprolactam structure thus we get the name
“Nylon-6”.
Complete step by step answer:
When caprolactam is heated at about 533 K in an inert atmosphere of nitrogen for about 4–5 hours, the ring breaks and undergoes polymerization. Later, the molten mass passes through spinnerets to form fibres of nylon 6. During polymerization, the amide bond within each caprolactam molecule is broken, with the active groups on each side re-forming two new bonds as the monomer becomes part of the polymer backbone.
As a synthetic fibre, Nylon 6 is generally white but can be dyed. It is known for its lustrous surface finish as well as its high impact strength and stress resistance effectively combining aesthetics with performance.
Note:
There is a quite similar polymer name nylon 6,6 which can cause confusion but they are two different polymers. Both Nylon 6 and 6,6 materials are easy to process, provide incredible strength, they’re very tough. While they’re very similar, each offers separate and distinct benefits. Nylon 6 is made from a caprolactam monomer having six carbon atoms whereas Nylon 6,6 is prepared from adipic acid and hexamethylenediamine. Unlike nylon 6,6, in which the direction of the amide bond reverses at each bond, all nylon 6 amide bonds lie in the same direction.
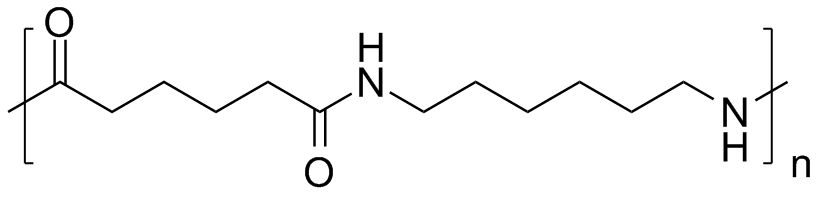
Structure of nylon 6,6.
-Nylon-6 is synthesised by ring opening polymerisation of caprolactam. There are six carbon atoms in the Caprolactam structure thus we get the name
“Nylon-6”.
Complete step by step answer:
When caprolactam is heated at about 533 K in an inert atmosphere of nitrogen for about 4–5 hours, the ring breaks and undergoes polymerization. Later, the molten mass passes through spinnerets to form fibres of nylon 6. During polymerization, the amide bond within each caprolactam molecule is broken, with the active groups on each side re-forming two new bonds as the monomer becomes part of the polymer backbone.
As a synthetic fibre, Nylon 6 is generally white but can be dyed. It is known for its lustrous surface finish as well as its high impact strength and stress resistance effectively combining aesthetics with performance.

Note:
There is a quite similar polymer name nylon 6,6 which can cause confusion but they are two different polymers. Both Nylon 6 and 6,6 materials are easy to process, provide incredible strength, they’re very tough. While they’re very similar, each offers separate and distinct benefits. Nylon 6 is made from a caprolactam monomer having six carbon atoms whereas Nylon 6,6 is prepared from adipic acid and hexamethylenediamine. Unlike nylon 6,6, in which the direction of the amide bond reverses at each bond, all nylon 6 amide bonds lie in the same direction.

Structure of nylon 6,6.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




