
Write down the IUPAC name of ${\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}}$.

Answer
611.7k+ views
Hint- Here, we will proceed by stating what IUPAC stands for. Then, we will discuss the general rules for IUPAC naming of a chemical compound having an alcohol group. Here, we will also be making the structure of the given compound.
Complete answer:
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC).
Since, the given chemical compound contains OH group which means it is an alcohol.
Following rules are followed for IUPAC naming of aldehydes:
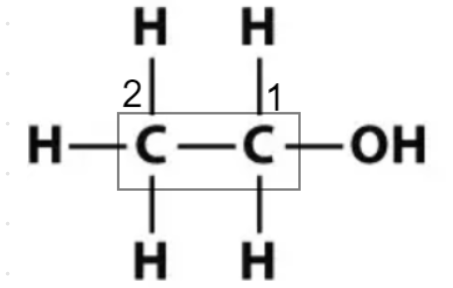
$1.$ Find the longest chain containing the hydroxy group (OH). If there is a chain with more carbons than the one containing the OH group it will be named as a substituent. Here, the longest chain or the parent chain is inside the rectangle as shown in the figure.
$2.$ Place the OH on the lowest possible number for the chain. With the exception of carbonyl groups such as ketones and aldehydes, the alcohol or hydroxy groups have first priority for naming. Here, the hydroxy group (OH) is attached to the carbon numbered 1 as shown in the figure.
$3.$ When naming a cyclic structure, the -OH group is assumed to be on the first carbon unless the carbonyl group is present, in which case the latter will get priority at the first carbon. When multiple -OH groups are on the cyclic structure, number the carbons on which the -OH groups reside. Here, this step is skipped because the given compound isn’t cyclic.
$4.$ Remove the final e from the parent alkane chain and add ol. When multiple alcohols are present use di, tri, etc before the ol, after the parent name. For example, 2,3-hexandiol. If a carbonyl group is present, the -OH group is named with the prefix hydroxy, with the carbonyl group attached to the parent chain name so that it ends with al (for aldehyde) or one (for ketone).
Here, there are two carbons, so we will use ethan and since carbon number 1 has –OH group attached on it, so ol will be used.
Therefore, the IUPAC name of ${\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}}$ is ethanol.
Note- In summary, the name of the compound is written out with the substituents in alphabetical order followed by the base name (derived from the number of carbons in the parent chain) followed by the main group attached (alcohol, aldehyde, etc). Commas are used between numbers and dashes are used between letters and numbers. There are no spaces in the name.
Complete answer:
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC).
Since, the given chemical compound contains OH group which means it is an alcohol.
Following rules are followed for IUPAC naming of aldehydes:
$1.$ Find the longest chain containing the hydroxy group (OH). If there is a chain with more carbons than the one containing the OH group it will be named as a substituent. Here, the longest chain or the parent chain is inside the rectangle as shown in the figure.
$2.$ Place the OH on the lowest possible number for the chain. With the exception of carbonyl groups such as ketones and aldehydes, the alcohol or hydroxy groups have first priority for naming. Here, the hydroxy group (OH) is attached to the carbon numbered 1 as shown in the figure.
$3.$ When naming a cyclic structure, the -OH group is assumed to be on the first carbon unless the carbonyl group is present, in which case the latter will get priority at the first carbon. When multiple -OH groups are on the cyclic structure, number the carbons on which the -OH groups reside. Here, this step is skipped because the given compound isn’t cyclic.
$4.$ Remove the final e from the parent alkane chain and add ol. When multiple alcohols are present use di, tri, etc before the ol, after the parent name. For example, 2,3-hexandiol. If a carbonyl group is present, the -OH group is named with the prefix hydroxy, with the carbonyl group attached to the parent chain name so that it ends with al (for aldehyde) or one (for ketone).
Here, there are two carbons, so we will use ethan and since carbon number 1 has –OH group attached on it, so ol will be used.
Therefore, the IUPAC name of ${\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}}$ is ethanol.
Note- In summary, the name of the compound is written out with the substituents in alphabetical order followed by the base name (derived from the number of carbons in the parent chain) followed by the main group attached (alcohol, aldehyde, etc). Commas are used between numbers and dashes are used between letters and numbers. There are no spaces in the name.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




