
Write the equation of reaction of chlorobenzene: Sulphonation
Answer
585.6k+ views
Hint: Chlorobenzene is an aromatic organic compound with the chemical formula ${C_6}{H_5}Cl$ .Further, the sulphonation refers to the introduction of sulphonic acid groups in benzene rings. When benzene is heated with fuming sulphuric acid or concentrated sulphuric acid, it yields benzene sulphonic acid.
Complete step by step answer:
The replacement of a hydrogen atom of an organic compound with a sulfonic acid often by the reaction with sulfuric acid at high temperatures is known as sulphonation.
Now, chlorobenzene was first described in 1851. It is manufactured by chlorination of benzene in the presence of a catalytic amount of Lewis acid such as ferric chloride, sulfur dichloride and anhydrous aluminum chloride.
Also, chlorobenzene undergoes sulphonation and the reaction is as shown:
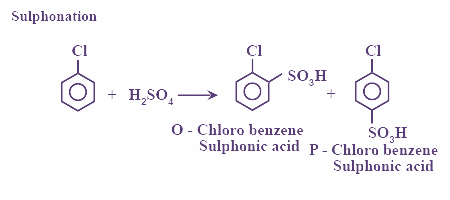
In this reaction, chlorobenzene reacts with sulfuric acid and gives o and p chlorobenzenesulfonic acid.
Further, the sulphonation of benzene is a reversible reaction. Sulphur trioxide readily reacts with water to produce sulphuric acid and heat.
Note:
As sulphonation is a reversible reaction, it can also be used in further substitution reactions in the form of a directing blocking group. The sulfonic group blocks the carbon from being attacked by other substituent and when the reaction is completed it can be further removed by reverse sulphonation.
Complete step by step answer:
The replacement of a hydrogen atom of an organic compound with a sulfonic acid often by the reaction with sulfuric acid at high temperatures is known as sulphonation.
Now, chlorobenzene was first described in 1851. It is manufactured by chlorination of benzene in the presence of a catalytic amount of Lewis acid such as ferric chloride, sulfur dichloride and anhydrous aluminum chloride.
Also, chlorobenzene undergoes sulphonation and the reaction is as shown:

In this reaction, chlorobenzene reacts with sulfuric acid and gives o and p chlorobenzenesulfonic acid.
Further, the sulphonation of benzene is a reversible reaction. Sulphur trioxide readily reacts with water to produce sulphuric acid and heat.
Note:
As sulphonation is a reversible reaction, it can also be used in further substitution reactions in the form of a directing blocking group. The sulfonic group blocks the carbon from being attacked by other substituent and when the reaction is completed it can be further removed by reverse sulphonation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




