
How can I write the formula for sodium sulfide?
Answer
560.7k+ views
Hint: The chemical formula is written by representing each atom in the given substance with their respective chemical symbols and satisfying the charge of each atom in such a way that the formed compound is having neutral charge. The chemical symbol or chemical representation of sodium is Na and the sulfide ion is having a charge of -2.
Complete answer:
In the given question, we are asked to write the chemical formula for sodium sulfide.
From the time we start studying about the chemical elements in the periodic table we study the chemical notations of the elements and we use these chemical notations for writing the equations. For some the chemical notations are the first letter or two letters in their name commonly used. And for some the chemical symbol is related to its Latin or Greek names.
The symbols will be written in capitalized font for the elements having one letter and for elements with two letters the first letter will be in capital form and the second letter will be in small letters.
Now let’s solve this question and write the solution for this question.
So first of all we should know the chemical symbols of sodium and sulfide.
The element sodium is represented as Na as it’s derived from natrium which is called as natron which means soda in English.
Sulfide is represented as S.
In the question the compound given is sodium sulfide, since sodium is a metallic element and sulfide is a non-metal the compound formed is an ionic compound, hence they will have charge and we have to nullify the charge as the compounds have zero charge.
Let’s see the charge of two atoms.
The sodium forms the monovalent sodium ion $\text{N}{{\text{a}}^{\text{+}}}$ i.e. it donates one electron while undergoing chemical combination whereas in sulfide ion they have a charge of -2 i.e. they accept two electrons during the chemical reaction.
Let’s represent the ions in the compounds as,
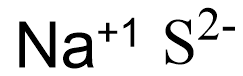
Here the charge is not balanced and to balance the charge of the compound let’s multiply a coefficient with the charge and write the multiplied number as the subscript of the respective element.
In the figure given above we could see that the sodium ion should be multiplied with 2, so that the charge is balanced and the compound charge becomes zero.
And the equation is,
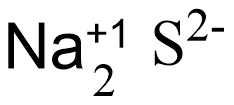
Hence, the final equation is $\text{N}{{\text{a}}_{\text{2}}}\text{S}$.
Note: If we know the charge and the chemical symbol of the atoms that take part in the chemical combination to form the compound, then we could directly find the equation by crisscross method by crisscrossing the charge of the atoms.
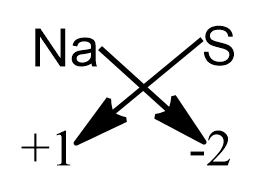
For finding the chemical equation through the crisscross method, we do not take into account the sign of the ion. Therefore the final equation obtained through the criss cross method is $\text{N}{{\text{a}}_{\text{2}}}\text{S}$.
Complete answer:
In the given question, we are asked to write the chemical formula for sodium sulfide.
From the time we start studying about the chemical elements in the periodic table we study the chemical notations of the elements and we use these chemical notations for writing the equations. For some the chemical notations are the first letter or two letters in their name commonly used. And for some the chemical symbol is related to its Latin or Greek names.
The symbols will be written in capitalized font for the elements having one letter and for elements with two letters the first letter will be in capital form and the second letter will be in small letters.
Now let’s solve this question and write the solution for this question.
So first of all we should know the chemical symbols of sodium and sulfide.
The element sodium is represented as Na as it’s derived from natrium which is called as natron which means soda in English.
Sulfide is represented as S.
In the question the compound given is sodium sulfide, since sodium is a metallic element and sulfide is a non-metal the compound formed is an ionic compound, hence they will have charge and we have to nullify the charge as the compounds have zero charge.
Let’s see the charge of two atoms.
The sodium forms the monovalent sodium ion $\text{N}{{\text{a}}^{\text{+}}}$ i.e. it donates one electron while undergoing chemical combination whereas in sulfide ion they have a charge of -2 i.e. they accept two electrons during the chemical reaction.
Let’s represent the ions in the compounds as,
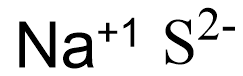
Here the charge is not balanced and to balance the charge of the compound let’s multiply a coefficient with the charge and write the multiplied number as the subscript of the respective element.
In the figure given above we could see that the sodium ion should be multiplied with 2, so that the charge is balanced and the compound charge becomes zero.
And the equation is,
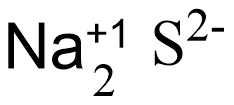
Hence, the final equation is $\text{N}{{\text{a}}_{\text{2}}}\text{S}$.
Note: If we know the charge and the chemical symbol of the atoms that take part in the chemical combination to form the compound, then we could directly find the equation by crisscross method by crisscrossing the charge of the atoms.
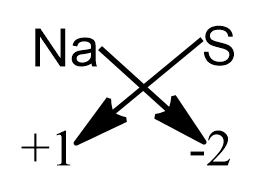
For finding the chemical equation through the crisscross method, we do not take into account the sign of the ion. Therefore the final equation obtained through the criss cross method is $\text{N}{{\text{a}}_{\text{2}}}\text{S}$.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




