
Write the IUPAC name of Ethyl methyl ketone.
Answer
573.3k+ views
Hint: According to IUPAC (International Union of Pure and Applied Chemistry), whenever we are going to write the IUPAC name of a compound, we have to give numbering first to functional groups or highly substituted carbon. Means lower numbering should be a functional group or highly substituted carbon present in the molecule or compound.
Complete answer:
- In the question it is given that to write the IUPAC name of Ethyl methyl ketone.
- First we have to write the structure of the given compound Ethyl methyl ketone.
- As per the name of the compound we can say that a ketone is present in the given molecule and an ethyl group is on one side and methyl group is on another side of the ketone should be attached.
- The structure of Ethyl methyl ketone is as follows.
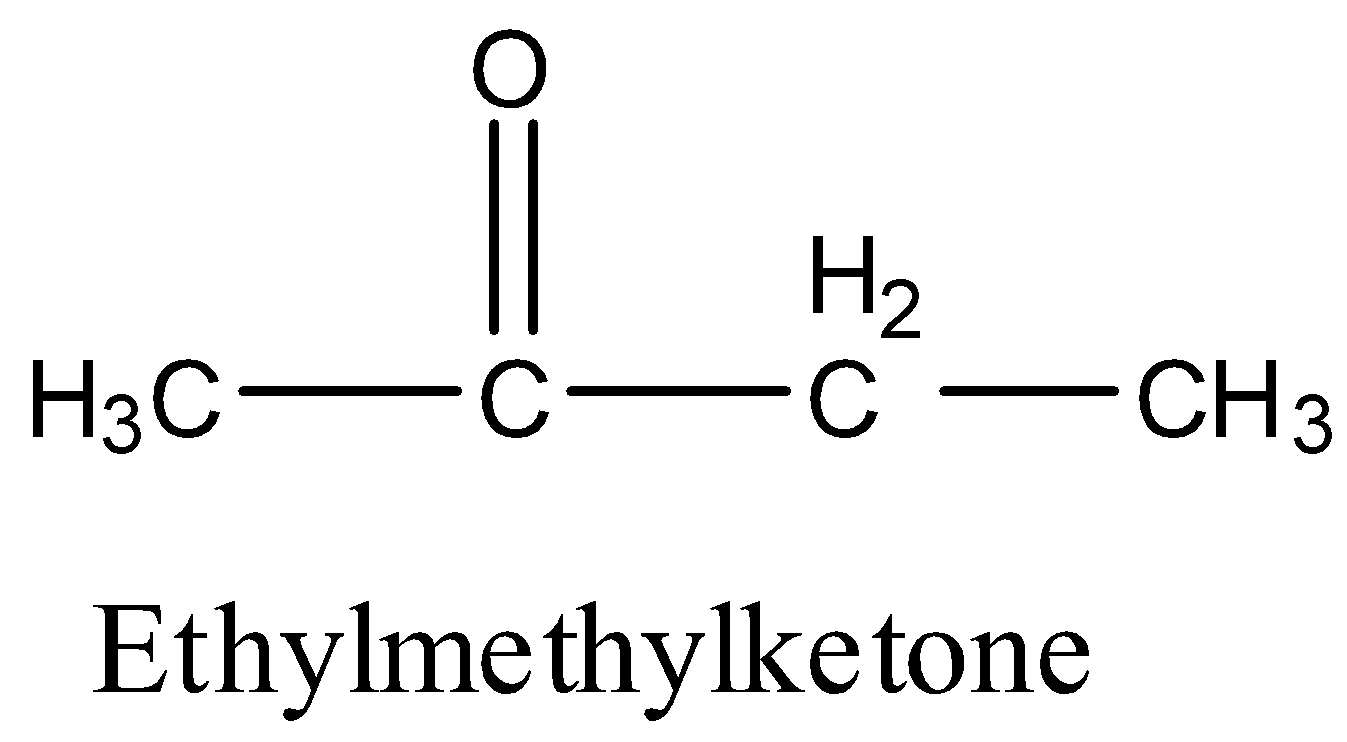
- We have to give numbering to all the carbon atoms in the given compound and the lowest number should come to the ketone functional group.
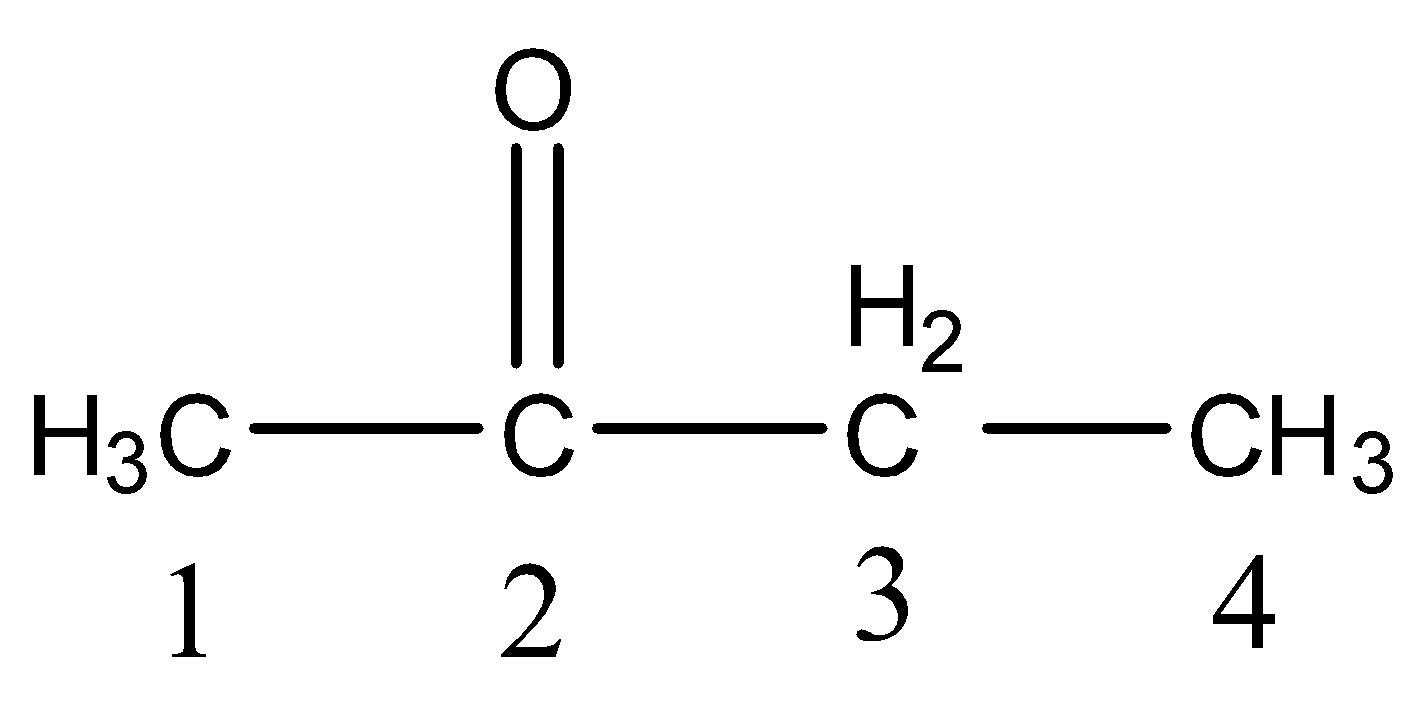
- There are four carbon atoms present in the chain then the name butane is supposed to be written in the IUPAC name.
- At carbon-2 there is a ketone functional group then we have to write 2-one.
- The IUPAC name of the given compound is Butan-2-one.
- Therefore the IUPAC name of Methyl Ethyl ketone is Butan-2-one.
Note: If we give numbering in a different direction, the name of the compound will be Butan-3-one. But the name Butan-3-one is wrong as per IUPAC rules. The functional group should get the least number while giving numbering to the carbons.
Complete answer:
- In the question it is given that to write the IUPAC name of Ethyl methyl ketone.
- First we have to write the structure of the given compound Ethyl methyl ketone.
- As per the name of the compound we can say that a ketone is present in the given molecule and an ethyl group is on one side and methyl group is on another side of the ketone should be attached.
- The structure of Ethyl methyl ketone is as follows.
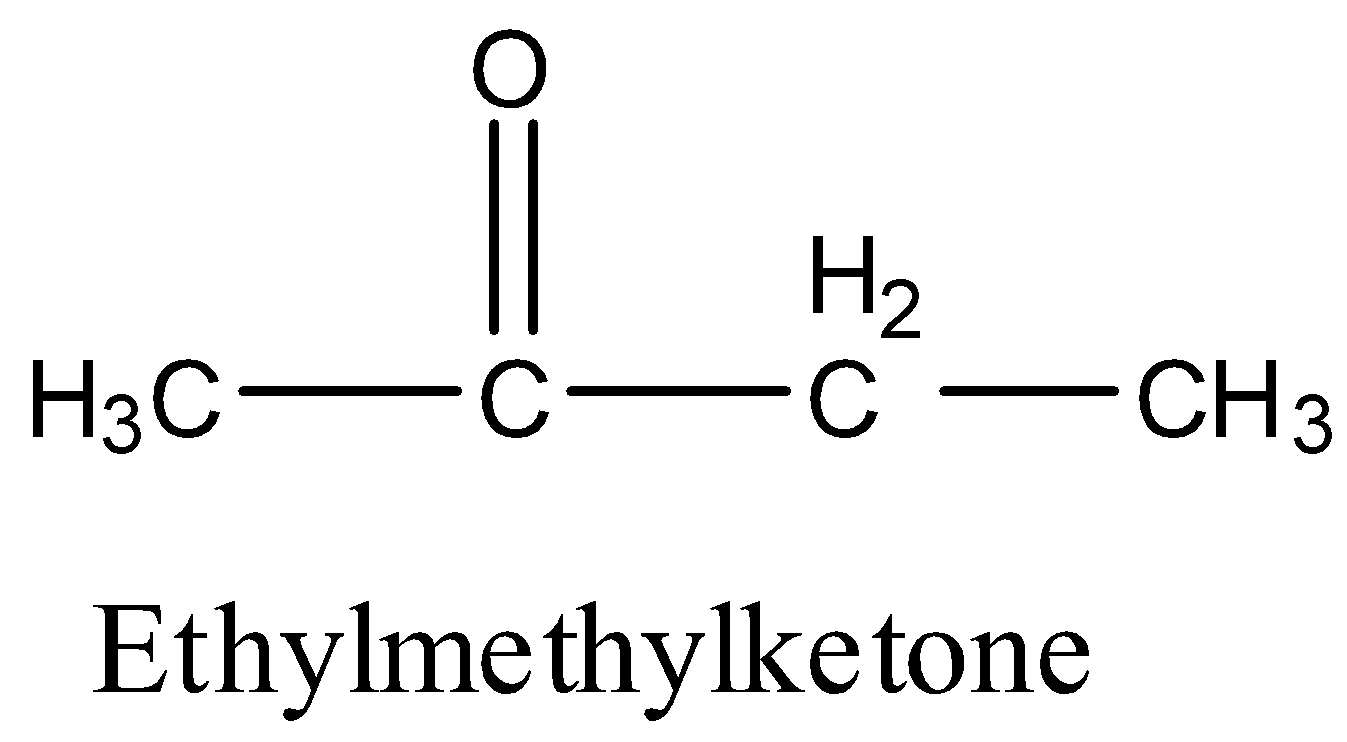
- We have to give numbering to all the carbon atoms in the given compound and the lowest number should come to the ketone functional group.

- There are four carbon atoms present in the chain then the name butane is supposed to be written in the IUPAC name.
- At carbon-2 there is a ketone functional group then we have to write 2-one.
- The IUPAC name of the given compound is Butan-2-one.
- Therefore the IUPAC name of Methyl Ethyl ketone is Butan-2-one.
Note: If we give numbering in a different direction, the name of the compound will be Butan-3-one. But the name Butan-3-one is wrong as per IUPAC rules. The functional group should get the least number while giving numbering to the carbons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




