
Write the IUPAC name of the compound?
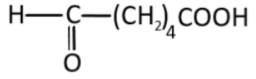
A. Hexanoic acid 5-al-1
B. 6-oxohexanoic acid
C. Hexanal-1-carboxylic acid
D. None of these
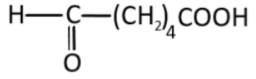
Answer
569.4k+ views
Hint:Recall some of the basic IUPAC nomenclature rules, and identify the two functional groups, these groups have carbonyl carbon (-c=o) and, the organic entity has a parent chain of 6 membered carbon, with the molecular formula ${{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{10}}}}{{\text{O}}_{\text{3}}}$
Complete answer:
Following the IUPAC (International union of pure and applied chemistry) nomenclature standards we will first identify the longest carbon chain and consider it as the parent chain,
Since it is straight-chain with no branching, the parent chain has 6 carbon atoms, therefore it is hexane (all carbon-carbon single bonds, hence -ane).
But it is not a simple hydrocarbon, the chain has two functional groups both having a carbonyl carbon group.
Now, we have to do the numbering to designate the position of the two groups in the carbon chain, but since there are two functional groups we will follow the priority order to give the lowest number to the most priority group, which is a carboxylic acid. This implies the 1 – oic acid, and 6 for the aldehyde group.
Therefore, we will use the suffix–oic acid (for carboxylic acid)
According to the priority order, We will consider the carboxylic acid as a part of the parent chain and aldehyde as a substitution in the parent chain.
The substituted aldehyde group (-CHO) will be named with the prefix ‘oxo’
All this information gives us the IUPAC name as 6-oxohexanoic acid
Hence, the correct answer is option (B) i.e., 6-oxohexanoic acid.
Additional information:
The priority order in functional groups follows the following order;
COOH, > \[{\text{ - S}}{{\text{O}}_{\text{3}}}{\text{H}}\], > -COOR (R= alkyl group), > -COCl, > \[ - {\text{CON}}{{\text{H}}_{\text{2}}}\], > -CN, > -HC=O, > >C=O, > -OH, > ${\text{ - N}}{{\text{H}}_{\text{2}}}$, > >C=C<, > -C-C-
Note:
As this organic compound has two functional groups, there could be 2-3 or even more functional group present in an organic compound, hence it is important to identify the priority group and the substituted group, because an aldehyde or a ketone belonging to the parent chain will be named by adding the suffix ‘al’ and ‘one’, but as a substituent, they would be named as ‘oxo’.
Complete answer:
Following the IUPAC (International union of pure and applied chemistry) nomenclature standards we will first identify the longest carbon chain and consider it as the parent chain,
Since it is straight-chain with no branching, the parent chain has 6 carbon atoms, therefore it is hexane (all carbon-carbon single bonds, hence -ane).
But it is not a simple hydrocarbon, the chain has two functional groups both having a carbonyl carbon group.
Now, we have to do the numbering to designate the position of the two groups in the carbon chain, but since there are two functional groups we will follow the priority order to give the lowest number to the most priority group, which is a carboxylic acid. This implies the 1 – oic acid, and 6 for the aldehyde group.
Therefore, we will use the suffix–oic acid (for carboxylic acid)
According to the priority order, We will consider the carboxylic acid as a part of the parent chain and aldehyde as a substitution in the parent chain.
The substituted aldehyde group (-CHO) will be named with the prefix ‘oxo’
All this information gives us the IUPAC name as 6-oxohexanoic acid
Hence, the correct answer is option (B) i.e., 6-oxohexanoic acid.
Additional information:
The priority order in functional groups follows the following order;
COOH, > \[{\text{ - S}}{{\text{O}}_{\text{3}}}{\text{H}}\], > -COOR (R= alkyl group), > -COCl, > \[ - {\text{CON}}{{\text{H}}_{\text{2}}}\], > -CN, > -HC=O, > >C=O, > -OH, > ${\text{ - N}}{{\text{H}}_{\text{2}}}$, > >C=C<, > -C-C-
Note:
As this organic compound has two functional groups, there could be 2-3 or even more functional group present in an organic compound, hence it is important to identify the priority group and the substituted group, because an aldehyde or a ketone belonging to the parent chain will be named by adding the suffix ‘al’ and ‘one’, but as a substituent, they would be named as ‘oxo’.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




