
Write the IUPAC names of:
A.Cyclo propyl chloride
B.Tert-amyl chloride
C.Isopropylidene dichloride
Answer
564.9k+ views
Hint:While writing the IUPAC name we need to always write the side substituent before the naming of the parent chain. We also need to specify on which carbon the side substituent is present.
Complete answer:
A.The compound A given to us is cyclo propyl chloride. The structure of this compound is as follow:
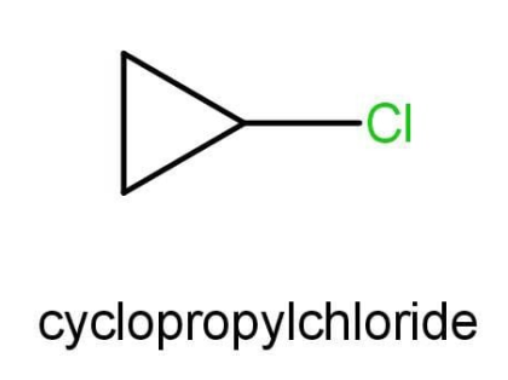
There is no functional group present in cyclopropyl chloride. This is a cyclic hydrocarbon and hence we will use the prefix cyclo. The side substituent is chlorine here for which we use the prefix choro. The cyclic carbon chain is of 3 carbons and for 3 carbons we use prop. This is a saturated hydrocarbon and hence we will use “ane” as a suffix. The IUPAC name will be:
1-chlorocyclopropane.
B.The compound B is Tert-amyl chloride. The structure is:
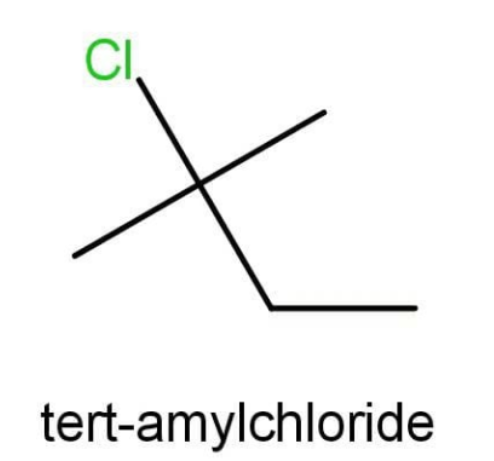
We need to select the longest linear carbon chain that contains the maximum number of carbons. Here a 4 carbons chain will be possible. There is no functional group but 2 side substituents are present that is chloro and methyl. They both are present on the carbon number 2. The molecule also contains only single bonds and hence is an alkane. The IUPAC name is:
2-chloro-2-methylbutane
C.The compound C is isopropylidene dichloride.
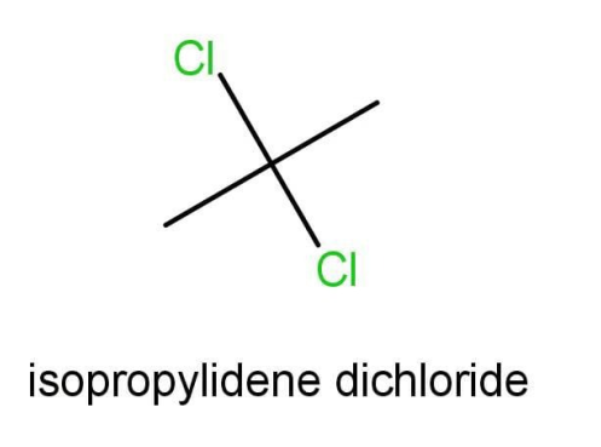
The parent chain consists of 3 carbons and hence we will use prop. There are two side substituents, both are chlorine and on the same position that is carbon number 2. The molecule also belongs to alkane. The IUPAC name can be written as:
2,2-dichloropropane
Note:
IUPAC stands for International Union for Pure and Applied chemistry. The IUPAC sets a standard for the naming of the organic compounds so that there is a single name accepted worldwide and no confusion in naming compounds occurs.
Complete answer:
A.The compound A given to us is cyclo propyl chloride. The structure of this compound is as follow:

There is no functional group present in cyclopropyl chloride. This is a cyclic hydrocarbon and hence we will use the prefix cyclo. The side substituent is chlorine here for which we use the prefix choro. The cyclic carbon chain is of 3 carbons and for 3 carbons we use prop. This is a saturated hydrocarbon and hence we will use “ane” as a suffix. The IUPAC name will be:
1-chlorocyclopropane.
B.The compound B is Tert-amyl chloride. The structure is:

We need to select the longest linear carbon chain that contains the maximum number of carbons. Here a 4 carbons chain will be possible. There is no functional group but 2 side substituents are present that is chloro and methyl. They both are present on the carbon number 2. The molecule also contains only single bonds and hence is an alkane. The IUPAC name is:
2-chloro-2-methylbutane
C.The compound C is isopropylidene dichloride.

The parent chain consists of 3 carbons and hence we will use prop. There are two side substituents, both are chlorine and on the same position that is carbon number 2. The molecule also belongs to alkane. The IUPAC name can be written as:
2,2-dichloropropane
Note:
IUPAC stands for International Union for Pure and Applied chemistry. The IUPAC sets a standard for the naming of the organic compounds so that there is a single name accepted worldwide and no confusion in naming compounds occurs.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




