
Write the mechanism of the reaction of HI with methoxy benzene.
Answer
519k+ views
Hint: The organic compound anisole, also known as methoxy benzene, has the formula. It's a colourless liquid with an anise seed aroma, and many of its derivatives can be used in natural and synthetic fragrances. The compound is mostly synthesised and serves as a precursor to other synthetic compounds.
Complete answer:
Methoxy benzene forms phenol and iodomethane as it reacts with hydroiodic acid HI.
We can represent this reaction as,
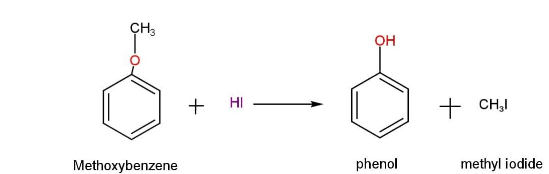
We know that methoxy benzene is alkyl aryl ether. So, methoxy benzene has two bonds that are joined by an oxygen atom. The oxygen-methyl group (O-alkyl bond) is one, and the oxygen-aryl group is the other (O-Aryl bond). Because of the partial double bond character of the Oxygen-Aryl bond, it is the most stable of the three.
That is, oxygen-methyl bond is cleaved in this reaction, resulting in methyl iodide and phenol.
The reaction between HI and methoxy benzene occurs in two stages and they are;
Stage 1: By trapping $H^+$ from HI, the oxygen in ether is protonated (added a proton), hence forming a protonated ether molecule known as oxonium ion.
Stage 2: Since Iodide ion (I-) is a strong nucleophile, the \[S{N_2}\] reaction occurs in this step. To form methyl iodide and phenol, the I- ion preferentially targets the protonated ether molecule's least substituted carbon (in this case, the methyl group).
Note:
We should also remember that phenol is an aromatic organic compound with the molecular formula. The chemical compound with the formula \[C{H_3}I\] is iodomethane, also known as methyl iodide and commonly abbreviated "MeI."
Complete answer:
Methoxy benzene forms phenol and iodomethane as it reacts with hydroiodic acid HI.
We can represent this reaction as,

We know that methoxy benzene is alkyl aryl ether. So, methoxy benzene has two bonds that are joined by an oxygen atom. The oxygen-methyl group (O-alkyl bond) is one, and the oxygen-aryl group is the other (O-Aryl bond). Because of the partial double bond character of the Oxygen-Aryl bond, it is the most stable of the three.
That is, oxygen-methyl bond is cleaved in this reaction, resulting in methyl iodide and phenol.
The reaction between HI and methoxy benzene occurs in two stages and they are;
Stage 1: By trapping $H^+$ from HI, the oxygen in ether is protonated (added a proton), hence forming a protonated ether molecule known as oxonium ion.
Stage 2: Since Iodide ion (I-) is a strong nucleophile, the \[S{N_2}\] reaction occurs in this step. To form methyl iodide and phenol, the I- ion preferentially targets the protonated ether molecule's least substituted carbon (in this case, the methyl group).
Note:
We should also remember that phenol is an aromatic organic compound with the molecular formula. The chemical compound with the formula \[C{H_3}I\] is iodomethane, also known as methyl iodide and commonly abbreviated "MeI."
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




