
Write the name of the monomer of the following polymer?
${[CO - {(C{H_2})_5} - NH]_n}$
Answer
573.9k+ views
Hint: Polymer is defined as that macromolecule which consists of a large number of the simple repeating structural units joined through a covalent bond in a regular pattern. And that simplest repeating unit is derived from simple and reactive molecules known as monomers.
Complete step by step answer:
The polymer given in the question is the nylon-6 and is also known as perlon. Nylon-6 is prepared from a single monomer known as caprolactam. Caprolactam on heating with water at high-temperature undergoes polymerization to give nylon-6. The monomer caprolactam used for the preparation of nylon-6 is prepared by cyclohexane. The preparation of caprolactam from cyclohexane is given as:
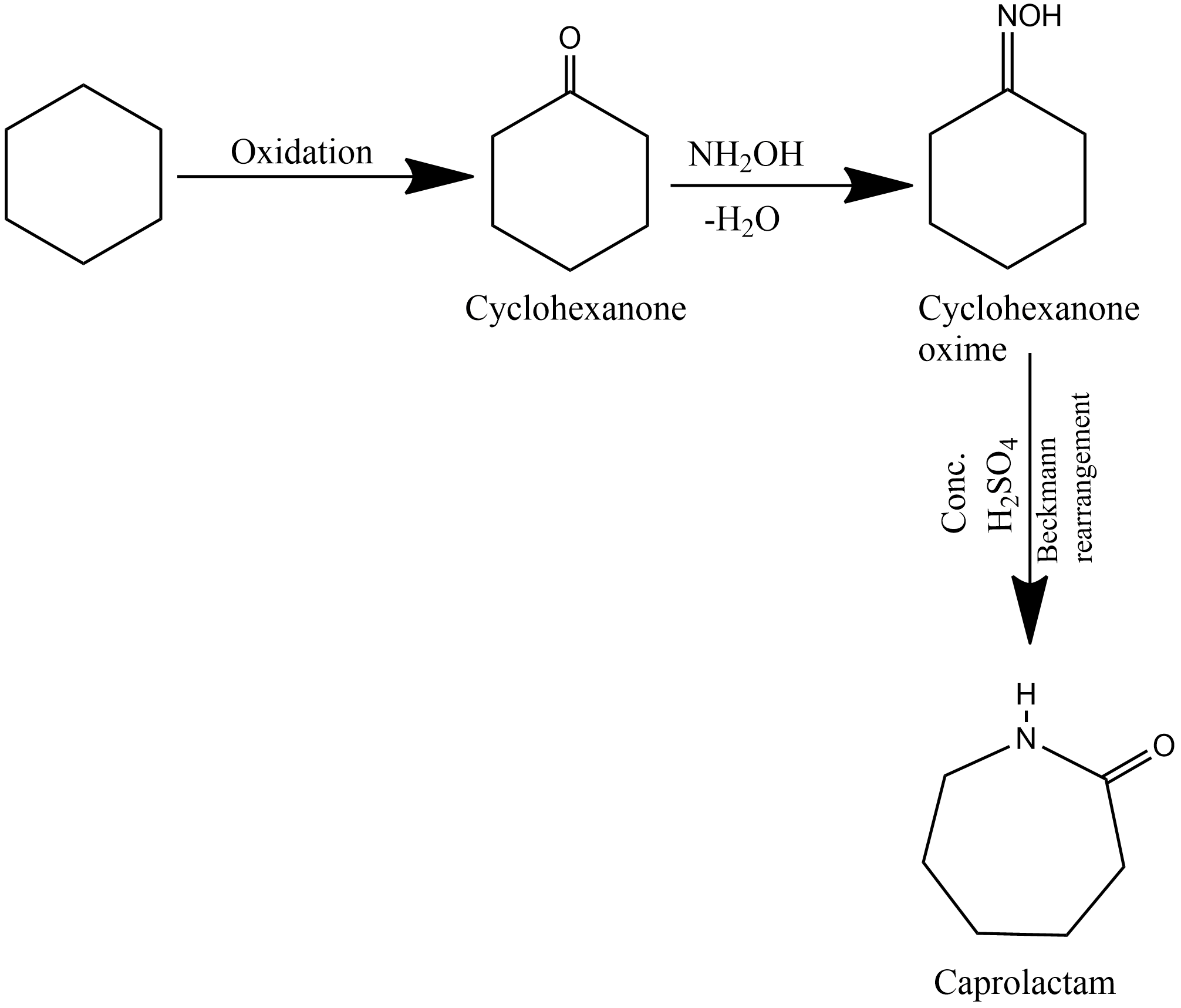
The caprolactam is then heated with the trace of water, it hydrolysis to ∈-aminocaproic acid which upon continuous heating undergoes polymerization and gives nylon-6. The chemical reaction for the polymerization of caprolactam is given as:
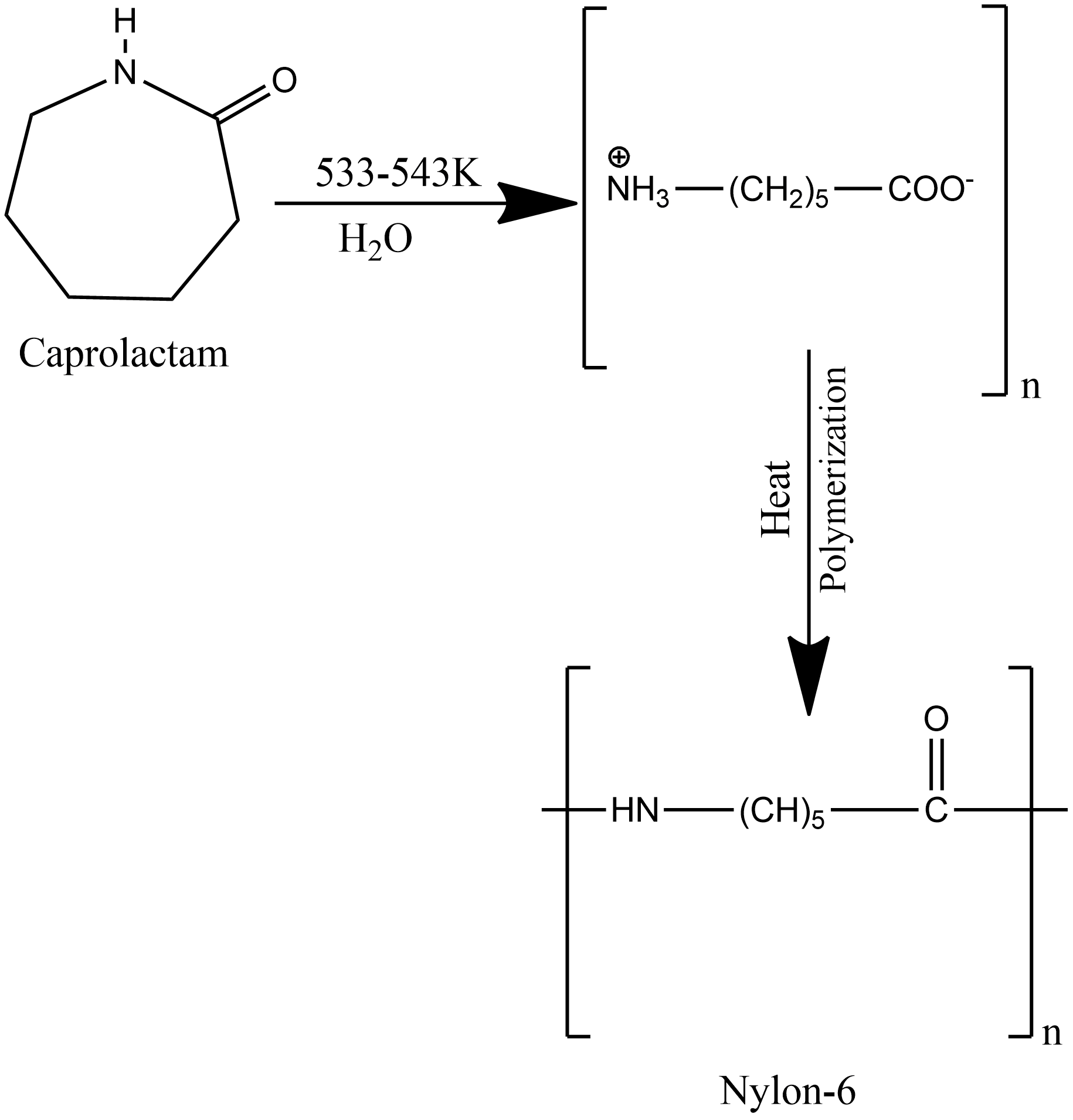
The fiber of nylon-6 is obtained when molten polymer is forced through a spinneret and the fiber formed is cooled by a stream of air.
Additional Information:
Nylon-6 contains amide linkage hence it is a type of polyamide. In the name Nylon, Ny is New York and lon is London as the most common form the nylon fibre i.e., nylon-6,6 was simultaneously prepared in New York and London. It is the type of condensation polymer.
Note: The polymer nylon-6 is nylon-six because of its monomer, i.e. caprolactam contains six carbon atoms. Nylon-6 is used for the manufacture of tyre cords, fabrics, and also for the manufacture of mountaineering ropes.
Complete step by step answer:
The polymer given in the question is the nylon-6 and is also known as perlon. Nylon-6 is prepared from a single monomer known as caprolactam. Caprolactam on heating with water at high-temperature undergoes polymerization to give nylon-6. The monomer caprolactam used for the preparation of nylon-6 is prepared by cyclohexane. The preparation of caprolactam from cyclohexane is given as:

The caprolactam is then heated with the trace of water, it hydrolysis to ∈-aminocaproic acid which upon continuous heating undergoes polymerization and gives nylon-6. The chemical reaction for the polymerization of caprolactam is given as:

The fiber of nylon-6 is obtained when molten polymer is forced through a spinneret and the fiber formed is cooled by a stream of air.
Additional Information:
Nylon-6 contains amide linkage hence it is a type of polyamide. In the name Nylon, Ny is New York and lon is London as the most common form the nylon fibre i.e., nylon-6,6 was simultaneously prepared in New York and London. It is the type of condensation polymer.
Note: The polymer nylon-6 is nylon-six because of its monomer, i.e. caprolactam contains six carbon atoms. Nylon-6 is used for the manufacture of tyre cords, fabrics, and also for the manufacture of mountaineering ropes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




