
Write the structural formula and names of all four possible aldol condensation products from ethanal and propanal.
Answer
592.2k+ views
Hint: Two molecules will be formed because of Self aldol condensation and two products will be formed from cross aldol condensation. The end product in aldol condensation is an alkene formed after release of water molecules.
Complete answer:
An aldol condensation is a reaction in which an enol or enolate ion of one molecule reacts with the carbonyl group of other molecule to form $\beta -hydroxyaldehyde$ or $\beta -hydroxyketone$,which is followed by dehydration to give a conjugated enone. It is a condensation reaction because it releases water molecules as a by-product. Aldol condensation can be both acid or base catalysed reaction. It involves carbanion as an intermediate. The requirement of aldol condensation is that the molecule must have a $\alpha -hydrogen$ in it.$\alpha -hydrogen$
Cross aldol is the condensation reaction between two molecules of different compounds of aldehyde or ketone, in presence of protic solvent like water or alcohol. When both the products undergoing aldol condensation have $\alpha -hydrogen$, then they react with themselves to form aldol product and the with other compounds to form the cross aldol product.
Ethanal is a two carbon containing aldehyde, with molecular formula ${{C}_{2}}{{H}_{4}}O$. The structure of ethanal is given as follows:
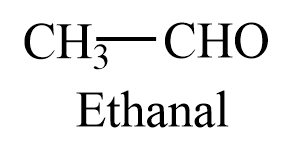
Propanal is a three carbon containing aldehyde, with molecular formula ${{C}_{3}}{{H}_{6}}O$. The structure of propanal is given as follows:
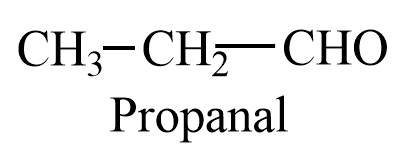
Both ethanal and propanal have $\alpha -hydrogen$, therefore, they will give aldol products with themselves and cross aldol products with each other.
Ethanal on aldol condensation gives But-2-enal as a product.
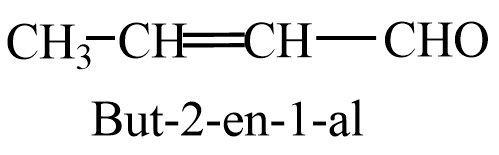
Propanal on aldol condensation gives 2-methylpent-2-enal.
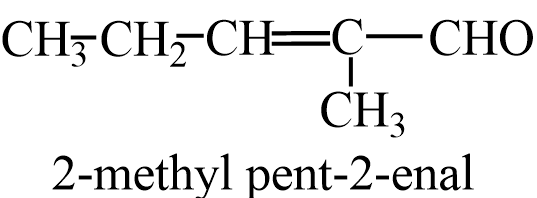
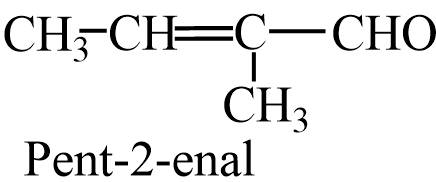
From cross aldol condensation of Ethanal and Propanal,it forms 2products, one when ethanol acts as enolate ion and one where propanal acts as enolate ion. The two cross aldol products are- 2-methylbut-2-enal and Pent-2-enal.
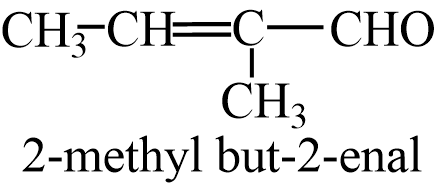
Note:
Ketone enolates are good nucleophiles,but the aldol reaction of ketones is usually not particularly successful.Also ketones are less reactive than aldehyde, so if the cross aldol is between aldehyde and ketone, aldehyde will be act as nucleophile.
Complete answer:
An aldol condensation is a reaction in which an enol or enolate ion of one molecule reacts with the carbonyl group of other molecule to form $\beta -hydroxyaldehyde$ or $\beta -hydroxyketone$,which is followed by dehydration to give a conjugated enone. It is a condensation reaction because it releases water molecules as a by-product. Aldol condensation can be both acid or base catalysed reaction. It involves carbanion as an intermediate. The requirement of aldol condensation is that the molecule must have a $\alpha -hydrogen$ in it.$\alpha -hydrogen$
Cross aldol is the condensation reaction between two molecules of different compounds of aldehyde or ketone, in presence of protic solvent like water or alcohol. When both the products undergoing aldol condensation have $\alpha -hydrogen$, then they react with themselves to form aldol product and the with other compounds to form the cross aldol product.
Ethanal is a two carbon containing aldehyde, with molecular formula ${{C}_{2}}{{H}_{4}}O$. The structure of ethanal is given as follows:
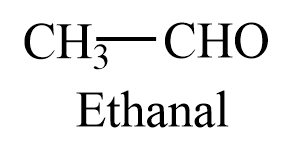
Propanal is a three carbon containing aldehyde, with molecular formula ${{C}_{3}}{{H}_{6}}O$. The structure of propanal is given as follows:
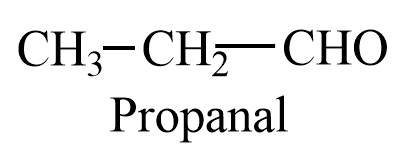
Both ethanal and propanal have $\alpha -hydrogen$, therefore, they will give aldol products with themselves and cross aldol products with each other.
Ethanal on aldol condensation gives But-2-enal as a product.
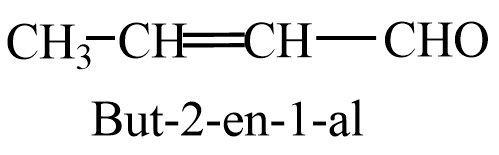
Propanal on aldol condensation gives 2-methylpent-2-enal.
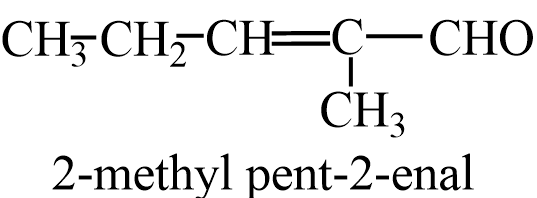
From cross aldol condensation of Ethanal and Propanal,it forms 2products, one when ethanol acts as enolate ion and one where propanal acts as enolate ion. The two cross aldol products are- 2-methylbut-2-enal and Pent-2-enal.
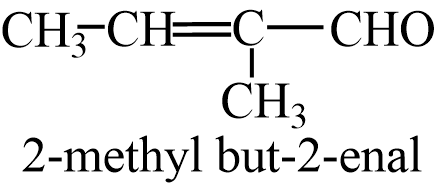

Note:
Ketone enolates are good nucleophiles,but the aldol reaction of ketones is usually not particularly successful.Also ketones are less reactive than aldehyde, so if the cross aldol is between aldehyde and ketone, aldehyde will be act as nucleophile.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




