
Write the structure of orthophosphorous acid and give its basicity.
Answer
583.8k+ views
Hint
Basicity is determined by the number of replaceable hydrogen atoms. If an acid contains 1 replaceable hydrogen, then its basicity is equal to 1 and known as monobasic. If an acid contains 2 replaceable hydrogen atoms, then its basicity is 2 and known as dibasic. And if an acid contains 3 replaceable hydrogen atoms then its basicity is 3 and known as tribasic.
Complete step by step solution
Orthophosphorous acid is also called phosphorous acid and has the formula ${{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}}$. It is also a mineral acid.
It is generally colourless and odourless. It is soluble in water and it forms a white crystals.it acts as a strong reducing agent and it can easily reduce many compounds like mercuric chloride to mercurous chloride, copper sulphate to metallic copper, silver nitrate to metallic silver, iodine to hydriodic acid etc.
Orthophosphorous acid when heated decomposes to give phosphoric acid and phosphine. It is prepared by the reaction of phosphorous trichloride and cold water. It is also prepared by the reaction of phosphorus trichloride and anhydrous oxalic acid.
The structure of Orthophosphorous acid is given below
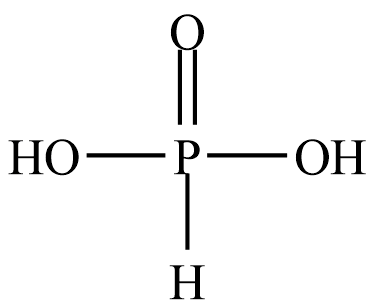
It can be seen that there are two $ - {\rm{OH}}$ groups present. There is a large electronegativity difference between the oxygen and hydrogen atoms. Due to which these two hydrogen atoms can easily remove. So, there are two numbers of replaceable hydrogen atoms.
Hence, its basicity is two.
Note:
Orthophosphorous acid is widely used in different industries namely the agricultural and pharmaceutical sector. It is used in the removal of rusting. It is also widely used in the food industry. It also helps to maintain the level of acid in food like meats, cheese etc. It is also used in various beauty products like dyes, nail products, make up etc.
Basicity is determined by the number of replaceable hydrogen atoms. If an acid contains 1 replaceable hydrogen, then its basicity is equal to 1 and known as monobasic. If an acid contains 2 replaceable hydrogen atoms, then its basicity is 2 and known as dibasic. And if an acid contains 3 replaceable hydrogen atoms then its basicity is 3 and known as tribasic.
Complete step by step solution
Orthophosphorous acid is also called phosphorous acid and has the formula ${{\rm{H}}_{\rm{3}}}{\rm{P}}{{\rm{O}}_{\rm{3}}}$. It is also a mineral acid.
It is generally colourless and odourless. It is soluble in water and it forms a white crystals.it acts as a strong reducing agent and it can easily reduce many compounds like mercuric chloride to mercurous chloride, copper sulphate to metallic copper, silver nitrate to metallic silver, iodine to hydriodic acid etc.
Orthophosphorous acid when heated decomposes to give phosphoric acid and phosphine. It is prepared by the reaction of phosphorous trichloride and cold water. It is also prepared by the reaction of phosphorus trichloride and anhydrous oxalic acid.
The structure of Orthophosphorous acid is given below

It can be seen that there are two $ - {\rm{OH}}$ groups present. There is a large electronegativity difference between the oxygen and hydrogen atoms. Due to which these two hydrogen atoms can easily remove. So, there are two numbers of replaceable hydrogen atoms.
Hence, its basicity is two.
Note:
Orthophosphorous acid is widely used in different industries namely the agricultural and pharmaceutical sector. It is used in the removal of rusting. It is also widely used in the food industry. It also helps to maintain the level of acid in food like meats, cheese etc. It is also used in various beauty products like dyes, nail products, make up etc.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




