
Xenon is noble gas but it forms compounds, Why? Draw the structure of any two compounds:
Answer
524.4k+ views
Hint :We know that a structural formula displays the atoms of the molecule in the order they are bonded. It can tell us about the central atom and the atoms bonded to it. As we know that the general electronic configuration of inert gases are \[n{{s}^{2}},n{{p}^{6}}\] except helium. Among the noble gas compounds, Xenon is known to form a number of compounds with electronegative elements like Fluorine and Oxygen.
Complete Step By Step Answer:
Let us recall some of the basic chemical properties of the noble gases like they possess the completely filled valence shell, high ionization enthalpy and more positive electron gain enthalpy. Xenon along with members of the group like helium, krypton, argon, neon and radon are termed as noble gas compounds. Among these Xenon is known to form a number of compounds with electronegative elements like Fluorine and Oxygen. Krypton forms very few compounds and only $Kr{{F}_{2}}$ is known. Even there are no true compounds of Argon, helium and Neon yet known.
In large sized Xenon, the electron attraction to the nucleus is weaker. It reacts with highly electronegative and small sized fluorine (and oxygen). Hence, the valence electron of \[Xe\] is attracted by fluorine (or oxygen). This helps in compound formation.
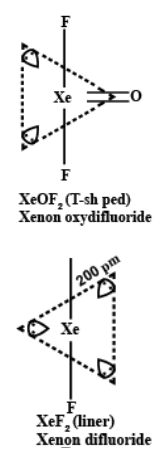
Note :
Remember that Try to remember the reagent in chemistry that is the basic need to be as good as anyone else in chemistry. And learn about the angles and configurations of the compound to make sure that if they have unpaired electrons or paired electrons which will be helpful in their bonding with other elements.
Complete Step By Step Answer:
Let us recall some of the basic chemical properties of the noble gases like they possess the completely filled valence shell, high ionization enthalpy and more positive electron gain enthalpy. Xenon along with members of the group like helium, krypton, argon, neon and radon are termed as noble gas compounds. Among these Xenon is known to form a number of compounds with electronegative elements like Fluorine and Oxygen. Krypton forms very few compounds and only $Kr{{F}_{2}}$ is known. Even there are no true compounds of Argon, helium and Neon yet known.
In large sized Xenon, the electron attraction to the nucleus is weaker. It reacts with highly electronegative and small sized fluorine (and oxygen). Hence, the valence electron of \[Xe\] is attracted by fluorine (or oxygen). This helps in compound formation.

Note :
Remember that Try to remember the reagent in chemistry that is the basic need to be as good as anyone else in chemistry. And learn about the angles and configurations of the compound to make sure that if they have unpaired electrons or paired electrons which will be helpful in their bonding with other elements.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




