NCERT Exemplar for Class 11 Chemistry - Classification of Elements and Periodicity in Properties - Free PDF Download
Free PDF download of NCERT Exemplar for Class 11 Chemistry Chapter 3 - Classification of Elements and Periodicity in Properties solved by expert Chemistry teachers on Vedantu.com as per NCERT (CBSE) Book guidelines. All Chapter 3 - Classification of Elements and Periodicity in Properties exercise questions with solutions to help you to revise the complete syllabus and score more marks in your examinations.
Elements and periodicity’s classification in properties is the most important chapter in Class 11 Chemistry. This chapter has been prescribed by the CBSE. To get a good percentage in the 12th board examination, the students should be thorough with the concepts that are taught in 11 standards as it is only then that a student can get a good score. The Class 11th Chemistry is not just useful for 12 standard board exams but it also forms the basis of National eligibility come entrance test/NEET examination. Students who want to pursue medical first have to clear their basic knowledge of the main subjects that are Chemistry, physics and biology. Learning the periodic table is the most important and the most basic foundation of Chemistry. It stands as a support system for all the new research and experiments that are held in many labs and it helps us to understand that the chemical elements live together in families. The third chapter is the Classification of elements and periodicity in properties in Class 11 Chemistry and this chapter teaches the historical development of the periodic table and the modern periodic law. It also explains in-depth how the periodic classification follows as a logical consequence of the electronic configuration of items. This chapter also examines the periodic trends in the physical and chemical properties of the elements.
Access NCERT Exemplar Solutions for Class 11 Chemistry Chapter – 3 Classification of Elements and Periodicity In Properties
Multiple Choice Questions (Type-I)
1. Consider the isoelectronic species, Na+, Mg2+, F– and O2–. The correct order of increasing length of their radii is _________.
(i) F- < O2– < Mg2+ < Na+
(ii) Mg2+ < Na+ < F– < O2–
(iii) O2– < F– < Na+ < Mg2+
(iv) O2– < F– < Mg2+ < Na+
Ans: (ii)
All the given ions are isoelectronic species thus their radii depend upon the charge more the negative charge higher would be the atomic radii and higher the positive charge lesser would be the atomic radii.
2. Which of the following is not an actinoid?
(i) Curium (Z = 96)
(ii) Californium (Z = 98)
(iii) Uranium (Z = 92)
(iv) Terbium (Z = 65)
Ans: (iv)
Actinoids possess Z=90-103.
Terbium is lanthanoid.
3. The order of screening effect of electrons of s, p, d and f orbitals of a given shell of an atom on its outer shell electrons is:
(i) s > p > d > f
(ii) f > d > p > s
(iii) p < d < s > f
(iv) f > p > s > d
Ans: (i)
The orbitals nearer to the nucleus possess more shielding effect.
4. The first ionisation enthalpies of Na, Mg, Al and Si are in the order:
(i) Na <Mg>Al<Si
(ii) Na>Mg>Al>Si
(iii) Na<Mg <Al<Si
(iv) Na>Mg>Al<Si
Ans: (i)
As Mg contains fully filled 3s orbital thus its ionisation is higher than that of Na and Al.
5. The electronic configuration of gadolinium (Atomic number 64) is
(i) (Xe) 4f35d56s2
(ii) (Xe) 4f75d26s1
(iii) (Xe) 4f75d16s2
(iv) (Xe) 4f85d66s2
Ans: (iii)
Gadolinium is a lanthanoid which belongs to the f-block elements with electronic configuration (n-2)f14(n-1)d0-1ns2
6. The statement that is not correct for periodic classification of elements is:
(i) The properties of elements are periodic function of their atomic numbers. (ii) Non metallic elements are less in number than metallic elements.
(iii) For transition elements, the 3d-orbitals are filled with electrons after 3p-orbitals and before 4s-orbitals.
(iv) The first ionisation enthalpies of elements generally increase with increase in atomic number as we go along a period.
Ans: (iii)
As per Aufbau principle 3d orbitals are filled first than 4s as due to lower n value.
7. Among halogens, the correct order of amount of energy released in electron gain (electron gain enthalpy) is:
(i) F > Cl > Br > I
(ii) F < Cl < Br < I
(iii) F < Cl > Br > I
(iv) F < Cl < Br < I
Ans: (i)
The electron gain enthalpy decreases down the group, however as the size of F atom is much smaller thus it leads to repulsion upon addition of electrons on its 2p orbital for this electron gain enthalpy of Cl is higher than that of F.
8. The period number in the long form of the periodic table is equal to
(i) magnetic quantum number of any element of the period.
(ii) atomic number of any element of the period.
(iii) maximum Principal quantum number of any element of the period.
(iv) maximum Azimuthal quantum number of any element of the period.
Ans: (iii)
Period number indicates the maximum value of principal quantum number.
9. The elements in which electrons are progressively filled in 4f-orbital are called
(i) actinoids
(ii) transition elements
(iii) lanthanoids
(iv) halogens
Ans: (iii)
The lanthanides which belong to the f-block elements are the elements where 4f-orbitals are filled progressively.
10. Which of the following is the correct order of size of the given species:
(i) I > I – > I +
(ii) I + > I – > I
(iii) I > I + > I –
(iv) I – > I > I +
Ans: (iv)
I+ possess the highest effective nuclear charge whereas I- possess the least among the given species.
11. The formation of the oxide ion, O2– (g), from oxygen atom requires first an exothermic and then an endothermic step as shown below:
O (g) + e– → O– (g) ; ∆ H = – 141 kJ mol–1
O– (g) + e– → O2– (g); ∆ H = + 780 kJ mol–1
Thus process of formation of O2– in gas phase is unfavourable even though O2– is isoelectronic with neon. It is due to the fact that,
(i) oxygen is more electronegative.
(ii) addition of electron in oxygen results in larger size of the ion.
(iii) electron repulsion outweighs the stability gained by achieving noble gas configuration.
(iv) O– ion has comparatively smaller size than oxygen atom.
Ans: (iii)
High amount of energy has to be supplied in order to overcome the e--e- repulsion that arises when O- gets converted to O2- by accepting an electron.
12. Comprehension given below is followed by some multiple choice questions. Each question has one correct option. Choose the correct option. In the modern periodic table, elements are arranged in order of increasing atomic numbers which is related to the electronic configuration. Depending upon the type of orbitals receiving the last electron, the elements in the periodic table have been divided into four blocks, viz, s, p, d and f. The modern periodic table consists of 7 periods and 18 groups. Each period begins with the filling of a new energy shell. In accordance with the Aufbau principle, the seven periods (1 to 7) have 2, 8, 8, 18, 18, 32 and 32 elements respectively. The seventh period is still incomplete. To avoid the periodic table being too long, the two series of f-block elements, called lanthanoids and actinoids are placed at the bottom of the main body of the periodic table.
(a) The element with atomic number 57 belongs to
(i) s-block
(ii) p-block
(iii) d-block
(iv) f-block
Ans: (iii) d-block
Lanthanum is the element with the atomic number 57 and it belongs to the d-block.
(b) The last element of the p-block in 6th period is represented by the outermost electronic configuration.
(i) 7s2 7p6
(ii) 5f 146d107s27p0
(iii) 4f145d106s26p6
(iv) 4f145d106s26p4
Ans: (iii) 4f145d106s26p6
The last element of the p-block in 6th period is Radon
(c) Which of the elements whose atomic numbers are given below, cannot be accommodated in the present set up of the long form of the periodic table?
(i) 107
(ii) 118
(iii) 126
(iv) 102
Ans:(iii) 126
Presently there are 7 periods and 18 groups in the long form of periodic table and it can acquire only 118 elements.
(d) The electronic configuration of the element which is just above the element with atomic number 43 in the same group is ________.
(i) 1s2 2s2 2p6 3s2 3p6 3d5 4s2
(ii) 1s2 2s2 2p6 3s2 3p6 3d5 4s3 4p6
(iii) 1s2 2s2 2p6 3s2 3p6 3d6 4s2
(iv) 1s2 2s2 2p6 3s2 3p6 3d7 4s2
Ans: (i) 1s2 2s2 2p6 3s2 3p6 3d5 4s2
Manganese is the element which is just above the element with atomic number 43
(e) The elements with atomic numbers 35, 53 and 85 are all ________.
(i) noble gases
(ii) halogens
(iii) heavy metals
(iv) light metals
Ans: (ii) halogens
Atomic number | Element |
35 | Bromine |
53 | Iodine |
85 | Astatine |
13. Electronic configurations of four elements A, B, C and D are given below : (A) 1s2 2s2 2p6 (B) 1s2 2s2 2p4 (C) 1s2 2s2 2p6 3s1 (D) 1s2 2s2 2p5
Which of the following is the correct order of increasing tendency to gain electron :
(i) A < C < B < D
(ii) A < B < C < D
(iii) D < B < C < A
(iv) D < A < B < C
Ans: (i)
The electron gain enthalpy of 2p orbital is higher than that of the 3s orbital.
Multiple Choice Questions (Type-II)
In the following questions two or more options may be correct.
14. Which of the following elements can show covalency greater than 4?
(i) Be
(ii) P
(iii) S
(iv) B
Ans: (ii) & (iii)
Both P and S have vacant d-orbital for which they execute extended covalency.
15. Those elements impart colour to the flame on heating in it, the atoms of which require low energy for the ionisation (i.e., absorb energy in the visible region of spectrum). The elements of which of the following groups will impart colour to the flame?
(i) 2
(ii) 13
(iii) 1
(iv) 17
Ans: (i)&(iii)
Alkali metals and alkaline earth metals have low ionization enthalpy.
16. Which of the following sequences contain atomic numbers of only representative elements?
(i) 3, 33, 53, 87
(ii) 2, 10, 22, 36
(iii) 7, 17, 25, 37, 48
(iv) 9, 35, 51, 88
Ans: (i)&(iv)
The s-block elements and p-block elements together are called representative elements.
17. Which of the following elements will gain one electron more readily in comparison to other elements of their group?
(i) S (g)
(ii) Na (g)
(iii) O (g)
(iv) Cl (g)
Ans: (i)&(iv)
Both S and Cl have the higher tendency to gain electrons in order to attain stable nearest noble gas configuration i.e. of Argon.
18. Which of the following statements are correct?
(i) Helium has the highest first ionisation enthalpy in the periodic table.
(ii) Chlorine has less negative electron gain enthalpy than fluorine.
(iii) Mercury and bromine are liquids at room temperature.
(iv) In any period, atomic radius of alkali metal is the highest.
Ans: (i),(iii)&(iv)
He with 1s2 electronic configuration has the highest electron gain enthalpy in the periodic table.
In any period the alkali metals have lowest effective nuclear charge in that particular period for which its atomic radius is highest in that period.
19. Which of the following sets contain only isoelectronic ions?
(i) Zn2+, Ca2+, Ga3+, Al3+
(ii) K+ , Ca2+, Sc3+, Cl –
(iii) P3–, S2–, Cl– , K+
(iv) Ti 4+, Ar, Cr3+, V5+
Ans: (ii)&(iii)
Isoelectronic species/ions are those species that possess the same number of electrons.
20. In which of the following options order of arrangement does not agree with the variation of property indicated against it?
(i) Al3+ < Mg2+ < Na+ < F– (increasing ionic size)
(ii) B < C < N < O (increasing first ionisation enthalpy)
(iii) I < Br < Cl < F (increasing electron gain enthalpy)
(iv) Li < Na < K < Rb (increasing metallic radius)
Ans: (ii)&(iii)
The ionization enthalpy of N is higher than that of F because it possess half filled orbital which provide it extra stability due to symmetry
As the atomic size of F is much smaller thus its electron gain enthalpy is lower than that of the Cl
21. Which of the following have no unit?
(i) Electronegativity
(ii) Electron gain enthalpy
(iii) Ionisation enthalpy
(iv) Metallic character
Ans: (i)&(iv)
Both electronegativity and metallic character do not possess units as they both are qualitative properties not a quantitative property.
22. Ionic radii vary in
(i) inverse proportion to the effective nuclear charge.
(ii) inverse proportion to the square of effective nuclear charge.
(iii) direct proportion to the screening effect.
(iv) direct proportion to the square of screening effect.
Ans: (i)&(iii)
Ionic radii decreases with the increase of the effective nuclear charge and increases with the increase of shielding effect or e--e- repulsion as it outweighs the effective nuclear charge effect.
23. An element belongs to 3rd period and group-13 of the periodic table. Which of the following properties will be shown by the element?
(i) Good conductor of electricity
(ii) Liquid, metallic
(iii) Solid, metallic
(iv) Solid, non metallic
Ans:(i)&(iii)
Aluminium is the element which is a metal and good conductor of electricity.
Short Answer Type
24. Explain why the electron gain enthalpy of fluorine is less negative than that of chlorine.
Ans: As the atomic size of F is much smaller which leads to high e--e- repulsion upon addition of electrons thus its electron gain enthalpy is less than that of Cl.
25. All transition elements are d-block elements, but all d-block elements are not transition elements. Explain.
Ans: Transition elements are named so because they form a bridge between s-block elements and p-block elements. Zn, Cd and Hg are among those elements that are d-block elements but they do not exhibit most of the properties of transition elements.
26. Identify the group and valency of the element having atomic number 119. Also predict the outermost electronic configuration and write the general formula of its oxide.
Ans:
Group | 1 |
Valency | 1 |
Outermost electronic configuration | 8s1 |
Formula of Oxide | M2O |
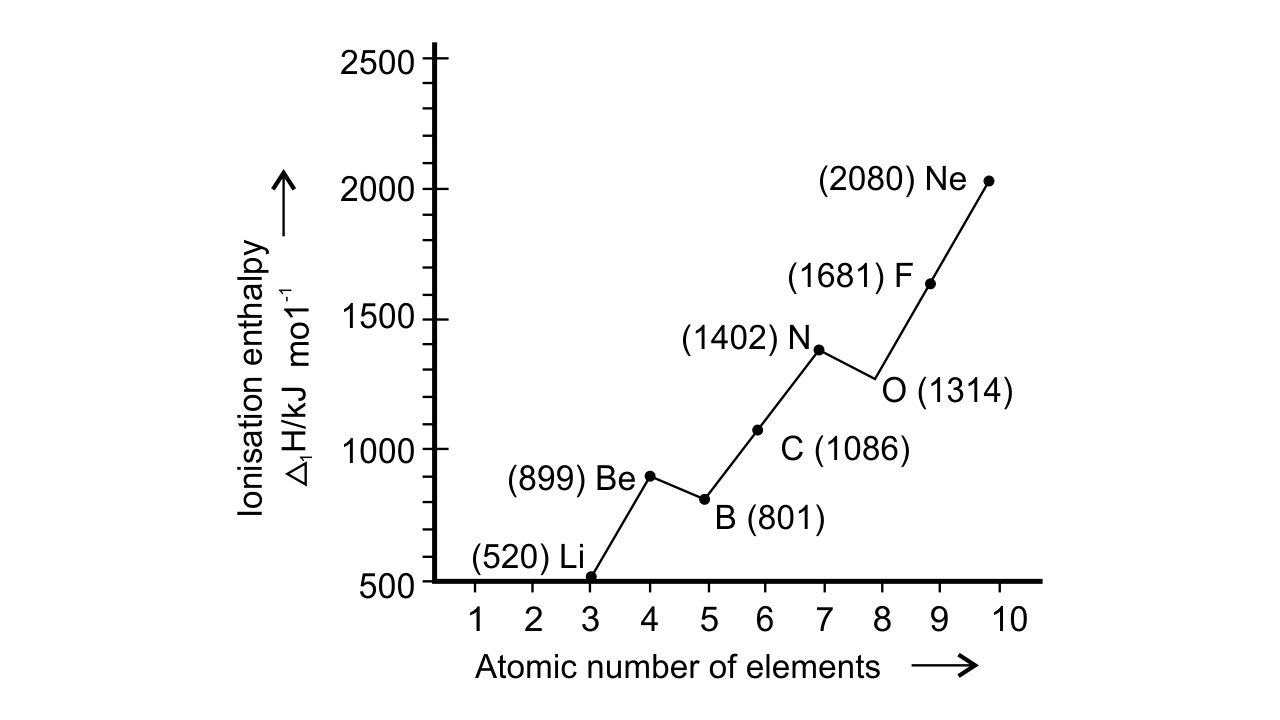
27.Ionisation enthalpies of elements of second period are given below : Ionisation enthalpy/ k cal mol–1 : 520, 899, 801, 1086, 1402, 1314, 1681, 2080.
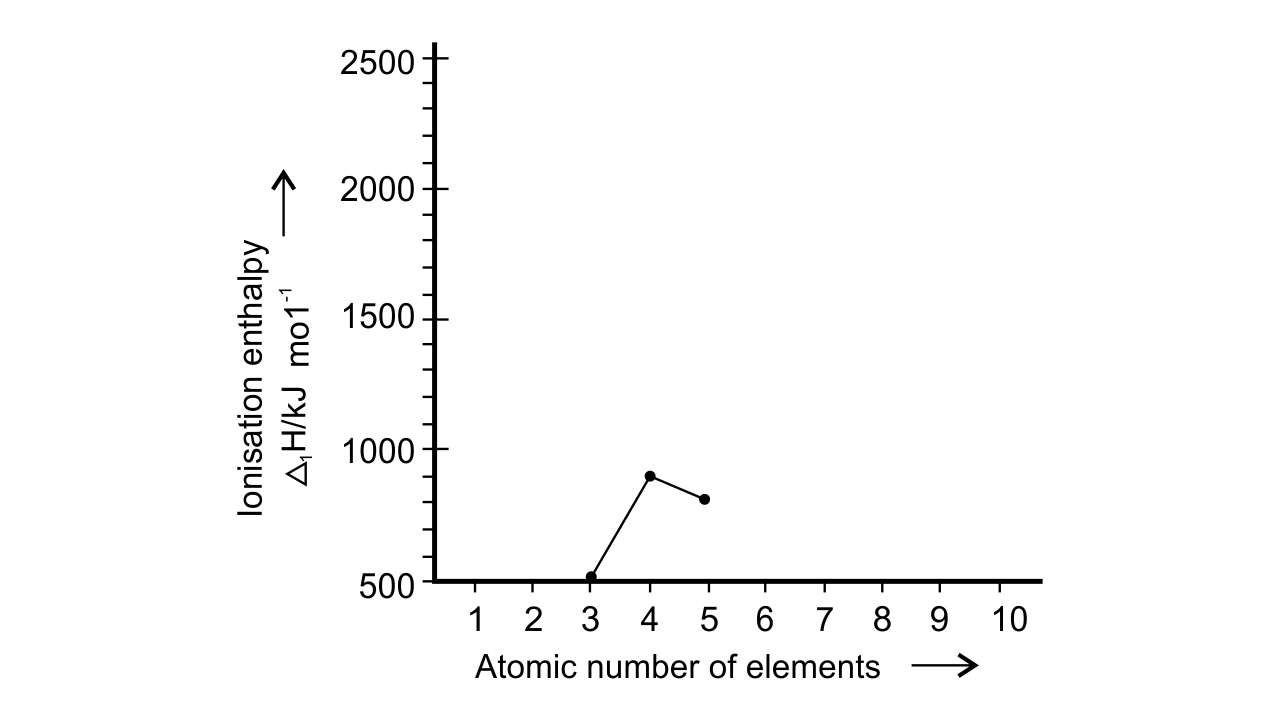
Match the correct enthalpy with the elements and complete the graph given in Fig. 3.1. Also write symbols of elements with their atomic number.
Ans:
28. Among the elements B, Al, C and Si,
(i) which element has the highest first ionisation enthalpy?
Ans: C has the highest ionization energy among the given elements as along the period ionization enthalpy increases whereas it decreases down the group.
(ii) which element has the most metallic character? Justify your answer in each case.
Ans: Al is the most metallic element among the given elements because down the group metallic character increases.
29. Write four characteristic properties of p-block elements
Ans: The characteristics properties of p-block elements are as follows:-
(i) It contain metals,nonmetals and metalloids
(ii) Mostly involved in covalent bonding
(iii) Some elements show variable oxidation states
(iv) It possesses relatively higher ionization enthalpy compared to the s-block elements.
30. Choose the correct order of atomic radii of fluorine and neon (in pm) out of the options given below and justify your answer.
(i) 72, 160
(ii) 160, 160
(iii) 72, 72
(iv) 160, 72
Ans: (i)
As across the period the atomic radius decreases due to the increase of effective nuclear charge
31. Illustrate by taking examples of transition elements and non-transition elements that oxidation states of elements are largely based on electronic configuration
Ans: The electronic configuration of Cr= 1s2 2s2 2p6 3s2 3p6 4s1 3d10
The electronic configuration of Cr after losing one electron= 1s2 2s2 2p6 3s2 3p6 3d10
The electronic configuration of F= 1s2 2s2 2p6 3s2 3p5
The electronic configuration of F after gaining one electron= 1s2 2s2 2p6 3s2 3p6
From the above electronic configuration we can see that chromium will achieve stable electronic configuration after losing one 4s electron and fluorine will achieve stable electronic configuration after gaining one electron. So, the oxidation of chromium will be +1 and that of fluorine will be -1.
32. Nitrogen has positive electron gain enthalpy whereas oxygen has negative. However, oxygen has lower ionisation enthalpy than nitrogen. Explain.
Ans: The N possesses a half filled p-orbital which provides it extra stability due to symmetry due to which its electron gain enthalpy is positive and its ionization enthalpy is larger than that of O.
33. First member of each group of representative elements (i.e., s and p-block elements) shows anomalous behaviour. Illustrate with two examples.
Ans: The anomalous behaviour of the first member of each group of representative elements i.e. of second period can be attributed to their small size, high charge/radius ratio, high electronegativity and absence of vacant d-orbitals to expand their oxidation state.The first member of each group of p-Block elements displays their greater ability to form multiple bond with itself ,e.g. C=C,O=O, N=N and to other second periodic elements, e.g. C=O,C=N,N=N.
34. p-Block elements form acidic, basic and amphoteric oxides. Explain each property by giving two examples and also write the reactions of these oxides with water.
Ans: In the p block, some elements are metallic some elements are non-metallic while some elements are metalloids in nature.
The oxides of metals are basic in nature and that of oxides of nonmetals are acidic in nature.
Acidic oxide
SO2 + H2O $\to $H2SO3
Basic oxide
Tl2O + 2HCl $\to $ 2TlCl +H2O
Amphoteric oxide
Al2O3 + 6HCl $\to $ 2AlCl3 + 3H2O
Al2O3 +2 NaOH $\to $ 2NaAlO2 + H2O
Reaction with water
\[{{\text{B}}_{\text{2}}}{{\text{O}}_{\text{3}}}\text{3}{{\text{H}}_{\text{2}}}\text{O}\to 2{{\text{H}}_{\text{3}}}\text{B}{{\text{O}}_{\text{3}}}\]
\[{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{11}}}\text{6}{{\text{H}}_{\text{2}}}\text{O}\to \text{4}{{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{4}}\]
\[\text{C}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{7}}}\text{+}{{\text{H}}_{\text{2}}}\text{O}\to \text{HCl}{{\text{O}}_{\text{4}}}\]
35. How would you explain the fact that the first ionisation enthalpy of sodium is lower than that of magnesium but its second ionisation enthalpy is higher than that of magnesium?
Ans: The electronic configuration of Na is (Ne)3s1 while that of Mg is (Ne)3s2 as Mg possesses a fully filled 3s orbital thus its first ionisation enthalpy is higher than that of Na.
After losing one electron Na obtained the electronic configuration of Ne while Mg acquired the electronic configuration of Na thus the second ionisation energy of Na is higher than that of Mg.
36. What do you understand about exothermic reactions and endothermic reactions? Give one example of each type.
Ans: Exothermic reaction- The reaction reaction in which heat is released.
For example- CaO + CO2 → CaCO3 + Heat
Endothermic reaction- The reaction in which heat is absorbed.
For example- 2NH3 + heat → N2 +3H2
37.Arrange the elements N, P, O and S in the order of-
(i) increasing first ionisation enthalpy.
(ii) increasing non metallic character.
Give reason for the arrangement assigned.
Ans: (i) S<P<O<N
The ionization enthalpy increases across the period and decreases down the group.
N possesses a half filled 2p orbital which provides it extra stability due to symmetry.
(ii)P<S<N<O
The non-metallic character increases across the period .
38. Explain the deviation in ionisation enthalpy of some elements from the general trend by using Fig. 3.2

Ans: The deviation in the ionization enthalpy of some elements from the general trend can be explained by the points as given below:-
(i) The fully filled and half filled orbital provide extra stability due to the symmetry
(ii) The effective nuclear charge
(iii) The e--e- repulsion which lead to instability
39. Explain the following:
(a) Electronegativity of elements increase on moving from left to right in the periodic table.
Ans: As the effective nuclear charge increases and shielding effect decreases across the periods thus the electronegativity increass on moving from left to right in the periodic table.
(b) Ionisation enthalpy decrease in a group from top to bottom?
Ans: As the number of shells increases down the group thus its ionisation enthalpy decreases down the group.
40. How does the metallic and non metallic character vary on moving from left to right in a period?
Ans: Metallic character- Tendency to lose electrons.
Non-Metallic character- Tendency to accept electrons.
Across the period the metallic character decreases whereas non-metallic character increases across the period.
41. The radius of Na+ cation is less than that of Na atom. Give reason.
Ans: The radius of Na+ ion is smaller than that of Na is due to the following reasons:-
(i) The effective nuclear charge of Na+ increases
(ii) The disappearance of 3s orbital from its outermost shell electronic configuration
42. Among alkali metals which element do you expect to be least electronegative and why?
Ans: The electronegativity of the alkali metals decreases down the group due to the increase of the shells thus Cs (Caesium) is the least electronegative metal in the alkali metals.
Matching Type
43. Match the correct atomic radius with the element.
Element | Atomic Radius (pm) |
Be | 74 |
C | 88 |
O | 111 |
B | 77 |
N | 66 |
Ans:
Be | 111 |
C | 77 |
O | 66 |
B | 88 |
N | 74 |
44. Match the correct ionisation enthalpies and electron gain enthalpies of the following elements.
Elements | ∆ H1 | ∆ H2 | ∆eg H | |
(i) Most reactive non metal | A | 419 | 3051 | – 48 |
(ii) Most reactive metal | B | 1681 | 3374 | – 328 |
(iii) Least reactive element | C | 738 | 1451 | – 40 |
(iv) Metal forming binary halide | D | 2372 | 5251 | + 48 |
Ans:
i | b |
ii | a |
iii | d |
iv | c |
45. Electronic configuration of some elements is given in Column I and their electron gain enthalpies are given in Column II. Match the electronic configuration with electron gain enthalpy.
Column (I) | Column (II) |
(i) \[\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{s}{{\text{p}}^{\text{6}}}\] | (a) -53 |
(ii) \[\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{s}{{\text{p}}^{\text{6}}}\] | (b)-328 |
(iii) \[\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{5}}}\] | (c)-141 |
(iv) \[\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{4}}}\] | (d)+48 |
Ans:
i | d |
ii | a |
iii | b |
iv | c |
Assertion and Reason Type
46. Assertion (A) : Generally, ionisation enthalpy increases from left to right in a period.
Reason (R) : When successive electrons are added to the orbitals in the same principal quantum level, the shielding effect of inner core of electrons does not increase very much to compensate for the increased attraction of the electron to the nucleus.
(i) Assertion is correct statement and reason is wrong statement.
(ii) Assertion and reason both are correct statements and reason is correct explanation of assertion.
(iii) Assertion and reason both are wrong statements.
(iv) Assertion is wrong statement and reason is correct statement.
Ans: (ii)
The ionization energy depends upon two factors
(a) The effective nuclear charge
(b) The e--e- repulsions
47. Assertion (A) : Boron has a smaller first ionisation enthalpy than beryllium. Reason (R) : The penetration of a 2s electron to the nucleus is more than the 2p electron hence 2p electron is more shielded by the inner core of electrons than the 2s electrons.
(i) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(ii) Assertion is correct statement but reason is wrong statement.
(iii) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(iv) Assertion and reason both are wrong statements.
Ans: (iii)
The Be has the electronic configuration of (He)2s2 while B has the electronic configuration of (He)2s22p1. As in Be the outermost orbital i.e. 2s is fully filled thus it provides extra stability to the Be compared to B.Apart from this 2s electron is more penetrated compared to the 2p electron for which 2p electron faces more shielding effect than that of 2s electron.
48. Assertion (A) : Electron gain enthalpy becomes less negative as we go down a group.
Reason (R) : Size of the atom increases on going down the group and the added electron would be farther from the nucleus.
(i) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(ii) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(iii) Assertion and reason both are wrong statements.
(iv) Assertion is wrong statement but reason is correct statement.
Ans: (ii)
The electron gain enthalpy decreases down the group due to the increase in the atomic size which leads to decrease in the effective nuclear charge.
Long Answer Type
49. Discuss the factors affecting electron gain enthalpy and the trend in its variation in the periodic table.
Ans: The factors that affects electron gain enthalpy are as follows:
(a) Effective nuclear charge with its increase the electron gain enthalpy also increases
(b) Atomic size with its increase the electron gain enthalpy decreases
(c) The e--e- repulsion with its increase the electron gain enthalpy decreases
(d) Whenever there exists half filled or completely filled orbital than it lead to decrease the electron gain enthalpy because it leads to give extra stability to the atom due to symmetry.
The electron gain enthalpy decreases down the group and increases across the period.
50. Define ionisation enthalpy. Discuss the factors affecting ionisation enthalpy of the elements and its trends in the periodic table.
Ans: The amount of energy required to remove an electron from the outermost shell of an isolated gaseous atom in its ground state is said to be ionisation enthalpy.
The factors that affect ionization enthalpy of the elements are as follows:
a. Effective nuclear charge with its increase the ionization enthalpy also increases
b. Atomic size with its increase the ionization enthalpy decreases
c. The e--e- repulsion with its increase the ionization enthalpy decreases
d. Whenever there exists half filled or completely filled orbital than it lead to increase the ionization enthalpy because it leads to give extra stability to the atom due to symmetry
The ionization enthalpy decreases down the group and increases across the period.
51. Justify the given statement with suitable examples— “the Properties of the elements are a periodic function of their atomic numbers”.
Ans: The chemical properties and physical properties of the elements depends upon its outer electron configuration which ultimately is the periodic function of the atomic number. The elements which possess the same outer electron configuration belong to the same group or family showing similar properties.
52. Write down the outermost electronic configuration of alkali metals. How will you justify their placement in group 1 of the periodic table?
Ans: The group 1 elements are called alkali metals which possess outermost electronic configuration as ns1.All the elements that have same outermost electron configuration possess similar properties and are placed in the same group.
Atomic Number | Symbol | Electronic Configuration |
3 | Li | \[\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{1}}}\text{ }\left( \text{or} \right)\text{ }\left[ \text{He} \right]\text{ 2}{{\text{s}}^{\text{1}}}\] |
11 | Na | \[\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{6}}}\text{3}{{\text{s}}^{\text{2}}}\text{ }\left( \text{or} \right)\text{ }\left[ \text{Ne} \right]\text{3}{{\text{s}}^{\text{1}}}\] |
19 | K | \[\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{6}}}\text{3}{{\text{s}}^{\text{2}}}\text{4}{{\text{s}}^{\text{1}}}\left( \text{or} \right)\text{ }\left[ \text{Ar} \right]\text{4}{{\text{s}}^{\text{1}}}\] |
37 | Rb | \[\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{6}}}\text{3}{{\text{s}}^{\text{2}}}\text{3}{{\text{d}}^{\text{10}}}\text{4}{{\text{s}}^{\text{2}}}\text{4}{{\text{p}}^{\text{6}}}\text{5}{{\text{s}}^{\text{1}}}\left( \text{or} \right)\left[ \text{Kr} \right]\text{5}{{\text{s}}^{\text{1}}}\] |
55 87 | Cs Fr | \[\text{1}{{\text{s}}^{\text{2}}}\text{2}{{\text{s}}^{\text{2}}}\text{2}{{\text{p}}^{\text{6}}}\text{3}{{\text{s}}^{\text{2}}}\text{3}{{\text{p}}^{\text{6}}}\text{3}{{\text{d}}^{\text{10}}}\text{4}{{\text{s}}^{\text{2}}}\text{4}{{\text{p}}^{\text{6}}}\text{5}{{\text{d}}^{\text{10}}}\text{5}{{\text{s}}^{\text{2}}}\text{5}{{\text{p}}^{\text{6}}}\text{6}{{\text{s}}^{\text{1}}}\left( \text{or} \right)\left[ \text{Xe} \right]\text{6}{{\text{s}}^{\text{1}}}\left[ \text{Rn} \right]\text{7}{{\text{s}}^{\text{1}}}\] |
53. Write the drawbacks in Mendeleev’s periodic table that led to its modification.
Ans: Mendeleev arranged the elements as the periodicity of their atomic weights.
The drawbacks of Mendeleev’s periodic table are as follows:-
a. The position of hydrogen in the periodic table is not specified
b. Isotopes are not included in the periodic table
c. Elements with higher atomic mass are placed before the elements with lower atomic mass. For e.g- Co & Ni
d. Gaps are left in his table considering the fact that more elements are yet to be discovered
e. Inappropriate position of group VII
54. In what manner is the long form of periodic table better than Mendeleev’s periodic table? Explain with examples
Ans: The following points make long form of periodic table better than Mendeleev’s periodic table :-
a. It is a periodic function of atomic number
b. Elements are grouped as per there outermost electronic configuration
c. Proper segregation of metals and non-metals
d. More appropriate position of group VII
55. Discuss and compare the trend in ionisation enthalpy of the elements of group1 with those of group17 elements
Ans: As we move across the periodic table the ionization energy increases because of the increase in the effective nuclear charge and decrease of the shielding effect as more and more electrons get added in the same orbital. Thus group 1 has lower ionization enthalpy compared to that of group 17 and also group 1 by losing one electron it will acquire the nearest noble gas electronic configuration which also contributes towards its lower ionization enthalpy.
Important Concepts Discussed in the Classification of Elements and Periodicity in Properties
Modern periodic law and the present form of the periodic table
Nomenclature of elements with atomic numbers > 100 and why do we need to classify elements?
Genesis of periodic classification
Electronic configurations of elements and the periodic table
Groupwise electronic configurations
Electronic configurations and types of elements: s-, p-, d-, f- BLOCKS
The s-block elements
The p – block elements
The d block elements
The f block elements (inner-transition elements)
Metals, non-metals, and metalloids
Periodic trends in properties of elements
Trends in physical properties
Atomic radius
Ionic radius
Ionization enthalpy
Electron gain enthalpy
Electronegativity
Periodic trends in chemical properties
Periodicity of valence or oxidation states
Anomalous properties of second-period elements
Periodic trends and chemical reactivity
Advantages of Referring to NCERT Books -
If a student wants to score 100% in their examinations then studying NCERT books is the most basic step of the learning process. The National Council of Educational Research and Training (NCERT), designs books that lay down in detail the basic concepts of every discipline Some of the main features are-
Even the most complex topics are written in a simplified language
NCERT is the foundation of understanding and learning the many concepts prescribed by the CBSE.
It includes many activities and exercises to keep the students engrossed.
The example problems in math and science assess the student’s analytical and critical thinking skills.
NCERT books adhere to the CBSE curriculum.
FAQs on NCERT Exemplar for Class 11 Chemistry Chapter-3 (Book Solutions)
1. Where can I find NCERT Exemplar solutions for Class 11 Chemistry - Classification of Elements and Periodicity in Properties?
Class 11 Chemistry NCERT exemplar solutions for the classification of elements and periodicity in properties can be easily found on Vedanta’s website. The solutions to these exemplar questions are easily available for free download. The students can download the free PDF and study in an offline environment without the unnecessary interruption of the Internet. Along with the NCERT exemplar solutions, students can also find relatable study material that will help them get a good score in the examination.
2. Why is Classification of Elements and Periodicity in Properties an important chapter?
Chapter 3 is extremely important as it explains in-depth the periodic table which forms the foundation of Chemistry. To score a good grade in the class 12 board examination students are advised to 1st clear the basic knowledge of Chemistry and what they can Do by studying the classification of elements and periodicity in properties.
3. Is it important to read the NCERT for Chemistry?
The NCERT books are prescribed by the Central Board of secondary education and it is extremely important to cover all the syllabus that is provided in the NCERT books. The NCERT books explain the difficult concepts in a much simpler language that can be understood by anyone. To get a good well Learning and reading from the NCERT book is the very first step that a student should take.
4. Who was Mendeleev? And what did he publish?
Dmitri Mendeleev was a scientist whose work has been acknowledged by scientists all over the world; he is generally credited with the development of the modern periodic table. It was Mendeleev who was responsible for publishing the periodic law for the first time, which says that the properties of the elements are a periodic function of their atomic weights.
5. Is the classification of Elements and Periodicity in Properties a difficult chapter?
The third chapter is extremely important; Chapter 8 forms the foundation of Chemistry. Difficult or not this chapter has to be studied in-depth as it deals with the periodic table which is an extremely important concept in the study of Chemistry. To make the learning process easier to export, teachers at Vedantu have curated all the study material and have also provided NCERT exemplar solutions so that students can keep in check of the progress and the study material and revision notes can also help in quick revision before the exam.

























