NCERT Exemplar for Class 11 Chemistry - Chemical Bonding and Molecular Structure - Free PDF Download
Free PDF download of NCERT Exemplar for Class 11 Chemistry Chapter 4 - Chemical Bonding and Molecular Structure solved by expert Chemistry teachers on Vedantu.com as per NCERT (CBSE) Book guidelines. All Chapter 4 - Chemical Bonding and Molecular Structure Exercise questions with solutions to help you to revise the complete syllabus and score more marks in your Examinations.
Summary of Chemical Bonding and Molecular Structure
Multiple Choice Questions (Type-I):
1. Isostructural species are those which have the same shape and hybridisation. Among the given species identify the isostructural pairs.
(i) $N{F_3}$ and $B{F_3}$
(ii) $B{F_4}^ -$ and $N{H_4}^ + $
(iii) $BC{l_3}$ and $BrC{l_3}$
(iv) $N{H_3}$ and $N{O_3}^ -$
Ans: In a molecular structure if a central atom has the same hybridization as the central atom of another molecular species, then the two structures are known as isostructural species.
(i) In ${\text{N}}{{\text{F}}_{\text{3}}}$ the central nitrogen atom is $s{p^3}$ hybridized and in ${\text{B}}{{\text{F}}_{\text{3}}}$ the central boron atom is $s{p^2}$ . So, these two molecules are not isostructural pairs.
(ii) In ${\text{B}}{{\text{F}}_{\text{4}}}^{\text{ - }}$ the central boron atom is $s{p^3}$ hybridized and in ${\text{N}}{{\text{H}}_{\text{4}}}^{\text{ + }}$ the central nitrogen atom is also $s{p^3}$ hybridized. So, these two molecules are identified as isostructural pairs.
(iii) In${\text{BC}}{{\text{l}}_{\text{3}}}$the central boron atom is $s{p^2}$ hybridized and in${\text{BrC}}{{\text{l}}_{\text{3}}}$the central bromine atom is $ s{p^3}d$ hybridized. So, these two molecules are not isostructural pairs.
(iv) In ${\text{N}}{{\text{H}}_{\text{3}}}$the central nitrogen atom is $s{p^3}$ and in${\text{N}}{{\text{O}}_{\text{3}}}^{\text{ - }}$the central nitrogen atom is $s{p^2}$ hybridized. So, these two molecules are not isostructural pairs.
Correct Answer: Option (ii)
2: Polarity in a molecule and hence the dipole moment depends primarily on electronegativity of the constituent atoms and shape of a molecule. Which of the following has the highest dipole moment?
(i) $C{O_2}$
(ii) $HI$
(ii) ${H_2}O$
(iii) $ S{O_2}$
Ans: The dipole moment of the molecule depends on the difference in the electronegativity of the atoms present in the structure. The dipole moment of ${\text{C}}{{\text{O}}_{\text{2}}}$ is $0$,${\text{HI}}$ is $0.38$ , ${{\text{H}}_{\text{2}}}{\text{O}}$ is $1.84$ and${\text{S}}{{\text{O}}_{\text{2}}}$ is $1.62$ .
As the oxygen atom is highly electronegative and hydrogen is least electronegative, the difference in electronegativity will be highest for water molecules. Therefore, water molecules will have the highest dipole moment.
Correct Answer: Option (iii)
3.The types of hybrid orbitals of nitrogen in$N{O_2}^ + ,N{O_3}^ -$ and$N{H_4}^ + $respectively are expected to be
(i) $sp,s{p^3}{\kern 1pt} {\kern 1pt} and{\kern 1pt} {\kern 1pt} s{p^2}$
(ii) $sp,s{p^2}{\kern 1pt} {\kern 1pt} and{\kern 1pt} {\kern 1pt} s{p^3}$
(iii) $s{p^2},sp{\kern 1pt} {\kern 1pt} and{\kern 1pt} {\kern 1pt} s{p^3}$
(iv) $s{p^2},s{p^3}{\kern 1pt} {\kern 1pt} and{\kern 1pt} {\kern 1pt} sp$
Ans:The hybrid orbitals of nitrogen in the given species will be confirmed by knowing the hybridization. In ${\text{N}}{{\text{O}}_{\text{2}}}^{\text{ + }}$ the central nitrogen atom is $sp$ hybridized as it has a linear shape. The molecule ${\text{N}}{{\text{O}}_3}^ -$ is $s{p^2}$ due to the presence of one lone pair of electrons on a nitrogen atom and hence having a bent geometry. The molecule ${\text{N}}{{\text{H}}_{\text{4}}}^{\text{ + }}$ is $s{p^3}$ hybridized having a tetrahedral geometry.
Correct Answer: Option (ii)
4.Hydrogen bonds are formed in many compounds e.g.,${H_2}O,HF,N{H_3}$The boiling point of such compounds depends to a large extent on the strength of hydrogen bonds and the number of hydrogen bonds. The correct decreasing order of the boiling points of above compounds is :
(i) $ HF > {H_2}O > N{H_3}$
(ii) ${H_2}O > HF > N{H_3}$
(iii) $ N{H_3} > HF > {H_2}O$
(iv) $ N{H_3} > {H_2}O > HF$
Ans:The size and the electronegativity are the main factors on which the strength of a compound depends on. As the size of the atom decreases, the electronegativity increases and thus the hydrogen-bonding becomes stronger. Thus, the strength of hydrogen-bonding in the given compounds is:
${{\text{H}}_{\text{2}}}{\text{O}} > {\text{HF}} > {\text{N}}{{\text{H}}_{\text{3}}}$
Correct Answer: Option (ii)
5. In$P{O_4}^{3 - }$ ion the formal charge on the oxygen atom of $P - O$ bond is
(i) $ + 1$
(ii) $ - 1$
(iii) $ - 0.75$
(iv) $ + 0.75$
Ans: In a polyatomic molecule or an ion, the formal charge of an atom is defined as the difference between the valence electrons present in that atom and the number of electrons assigned to that atom in the Lewis structure.
Formal charge = No. of valence electrons $-$ (No. o f lone pair $ + \dfrac{1}{2}{N_b}$ electrons)
Formal Charge $ = 6 - \left( {6 + \dfrac{1}{2} \times 2} \right)$
Formal Charge $ = - 1$
Correct Answer: Option (ii)
6. In$N{O_3}^ -$ion, the number of bond pairs and lone pairs of electrons on nitrogen atom are
(i) $2,2$
(ii) $3,1$
(iii) $1,3$
(iv) $4,0$
Ans: The number of bond pairs on the nitrogen atom of ${\text{N}}{{\text{O}}_{\text{3}}}^{\text{ - }}$ are $4$ and the structure of ${\text{N}}{{\text{O}}_{\text{3}}}^{\text{ - }}$ does not have any lone pairs of electrons. The structure of ${\text{N}}{{\text{O}}_{\text{3}}}^{\text{ - }}$ molecule is:
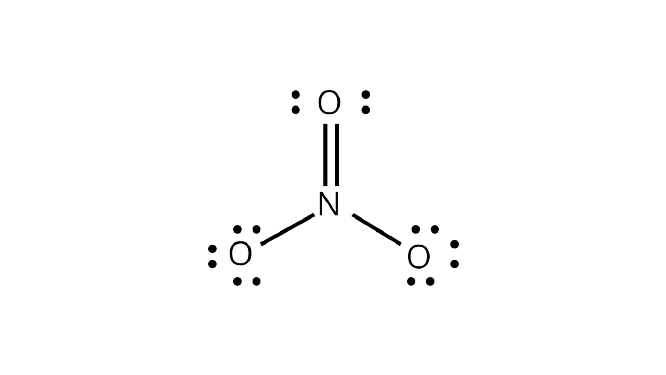
Correct Answer: Option (iv)
7. Which of the following species has tetrahedral geometry?
(i) $ B{H_4}^ -$
(ii) $ N{H_2}^ -$
(iii) $ C{O_3}^{2 - }$
(iv) ${H_3}{O^ + }$
Ans: (i)The molecule of ${\text{B}}{{\text{H}}_4}^ -$ has four bond pairs and zero lone pair of electrons, so it will be a tetrahedral molecule.
(ii)${\text{N}}{{\text{H}}_2}^ -$ has two bond pairs and two lone pairs of electrons on the nitrogen atom. So, it will have a bent geometry.
(iii)${\text{C}}{{\text{O}}_3}^{2 - }$ has three bond pairs and no lone pairs of electrons on carbon atoms. So, it will have a trigonal planar geometry.
(iv)${{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}$ has three bond pairs and one lone pair of electrons on oxygen atoms. So, it has a pyramidal geometry.
Correct Answer: Option (i)
8. Number of $\Pi $ bonds and$\sigma $bonds in the following structure is–
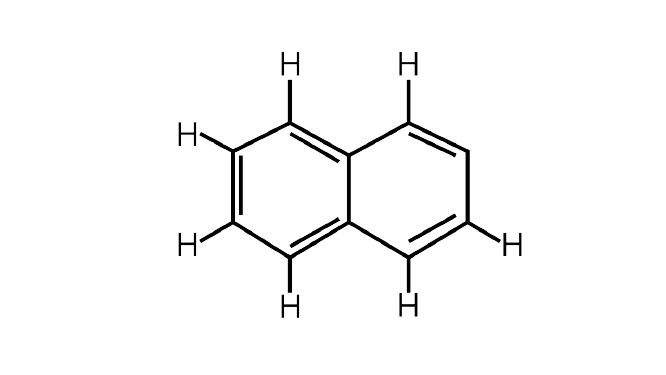
(i) $6,19$
(ii) $4,20$
(iii) $5,19$
(iv) $5,20$
Ans: A double bond has one$\sigma $bond and one$\Pi $bond. In the above given structure$5\Pi $ bonds are present. The$\sigma $bonds are the additions of all the single bonds in the structure. There are $19\sigma $ bonds in the above given structure.
Correct Answer: Option (iii)
9. Which molecule/ion out of the following does not contain unpaired electrons?
(i) ${N_2}^ + $
(ii) ${O_2}$
(iii) ${O_2}^{2 - }$
(iv) ${B_2}$
Ans: The electronic configurations of the given molecules/ions are:
(i) ${{\text{N}}_{\text{2}}}^{\text{ + }}$has $13$ electrons in its structure and its molecular electronic configuration is: $\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_x}^2 = \Pi 2{p_y}^2,\Pi 2{p_z}^1$ . Hence the ion of ${{\text{N}}_{\text{2}}}^{\text{ + }}$has one unpaired electron in $\Pi 2{p_z}^1$ sub-shell.
(ii) ${{\text{O}}_{\text{2}}}$has 16 electron in its structure and its molecular electronic configuration is: $\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_x}^2 = \Pi 2{p_y}^2,\Pi 2{p_z}^2 = {\Pi ^*}2{p_x}^1,{\Pi ^*}2{p_y}^1$. Hence, the molecule of ${{\text{O}}_{\text{2}}}$has two unpaired electrons in its structure.
(iii) ${{\text{O}}_2}^{2 - }$has $18$ electrons in its structure and its molecular electronic configuration is: $\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_x}^2 = \Pi 2{p_y}^2,\Pi 2{p_z}^2 = {\Pi ^*}2{p_x}^2,{\Pi ^*}2{p_y}^2$. Hence, the peroxide ion ${{\text{O}}_2}^{2 - }$does not contain any unpaired electrons.
(iv) ${{\text{B}}_{\text{2}}}$has $10$electrons in its structure and its electronic configuration is: $\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2} = \Pi 2{p_y}^1,\Pi 2{p_z}^1$. Hence the structure of boron has two unpaired electrons.
Correct Answer: Option (iii)
10. In which of the following molecule/ion all the bonds are not equal?
(i) $ Xe{F_4}$
(ii) $ B{F_4}^ -$
(iii) ${C_2}{H_4}$
(iv) $ Si{F_4}$
Ans: (i) The molecule ${\text{Xe}}{{\text{F}}_{\text{4}}}$has a square planar geometry and the bond lengths of all the fluoride atoms attached to xenon atoms are equal.
(ii) The molecule${\text{B}}{{\text{F}}_{\text{4}}}^ -$has a tetrahedral geometry and the bond lengths of all the fluoride bonds attached to boron are equal.
(iii) In the molecule ${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}$all the bonds are not equal. The bond length of the double bond between the carbon atoms is $134{\kern 1pt} {\kern 1pt} {\kern 1pt} {\text{pm}}$ and the bond length of ${\text{C}} - {\text{H}}$ is $110{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\text{pm}}$.
(iv) The molecule ${\text{Si}}{{\text{F}}_{\text{4}}}$ has a tetrahedral geometry and the bond lengths of all the fluoride atoms attached to silicon atoms are equal.
Correct Answer: Option (iii)
11. In which of the following substances will hydrogen bond be strongest?
(i) $ HCl$
(ii) ${H_2}O$
(iii) $ HI$
(iv) ${H_2}S$
Ans:Hydrogen bond is strongest in that molecules have a higher difference in electronegativity. Due to the small size of the oxygen atom, it has the highest electronegative character. So, ${{\text{H}}_{\text{2}}}{\text{O}}$molecules will have the strongest hydrogen bonding.
${\text{HCl}}$,${\text{HI}}$ and${{\text{H}}_{\text{2}}}{\text{S}}$ do not have hydrogen bonding between their atoms. So, ${{\text{H}}_{\text{2}}}{\text{O}}$ will have the strongest hydrogen bonding.
Correct Answer: Option (ii)
12. If the electronic configuration of an element is\[1{s^2} 2{s^2} 2{p^6} 3{s^2}3{p^6} 3{d^2} 4{s^2}\] , the four electrons involved in chemical bond formation will be_____.
(i) $3{p^6}$
(ii) $3{p^6},4{s^2}$
(iii) $3{p^6},3{d^2}$
(iv) $3{d^2},4{s^2}$
Ans:The electrons involved in the formation of any chemical bond are the valence shell electrons. In the given electronic configuration \[1{s^2}{\text{ }}2{s^2}{\text{ }}2{p^6}{\text{ }}3{s^2}3{p^6}{\text{ }}3{d^2}{\text{ }}4{s^2}\], the valence shell electrons are in d-orbital and s-orbital. So, the four electrons involved in the chemical bond formation will be $3{d^2},4{s^2}$.
Correct Answer: Option (iv)
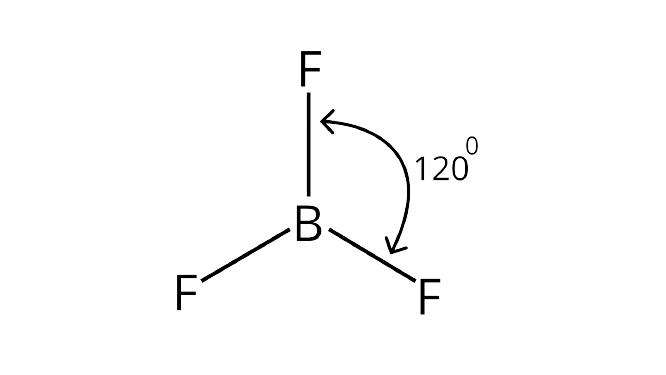
13. Which of the following angle corresponds to $s{p^2}$ hybridisation?
(i) $9{0^o}$
(ii) $12{0^o}$
(iii) $18{0^o}$
(iv) $10{9^o}$
Ans:For a molecule having a hybridization of $s{p^2}$, its geometry in plane is trigonal planar. For a molecule having a trigonal planar geometry, its bond angle will be ${120^ \circ }$
The electronic configurations of three elements, A, B and C are given below. Answer the questions 14 to 17 on the basis of these configurations.
${\text{A}}$\[1{s^2}{\text{ }}2{s^2}{\text{ }}2{p^6}\]
${\text{B}}$\[1{s^2}{\text{ }}2{s^2}{\text{ }}2{p^6}{\text{ }}3{s^2}{\text{ }}3{p^3}\]
${\text{C}}$\[1{s^2}{\text{ }}2{s^2}{\text{ }}2{p^6}{\text{ }}3{s^2}{\text{ }}3{p^5}\]
Correct Answer: Option (ii)
14. Stable form of A may be represented by the formula:
(i) $A$
(ii) ${A_2}$
(iii) ${A_3}$
(iv) ${A_4}$
Ans: The electronic configuration of ${\text{A}}$ is\[1{s^2}{\text{ }}2{s^2}{\text{ }}2{p^6}\] having no unpaired electrons in the outer most shells. So, an atom having no unpaired electron in its valence shell is said to be stable.
Correct Answer: Option (i)
15. Stable form of C may be represented by the formula :
(i) $ C$
(ii) ${C_2}$
(iii) ${C_3}$
(iv) ${C_4}$
Ans: The electronic configuration of ${\text{C}}$ is given as\[1{s^2}{\text{ }}2{s^2}{\text{ }}2{p^6}{\text{ }}3{s^2}{\text{ }}3{p^5}\] which the electronic configuration of chlorine atom (${\text{Cl}}$). It is unstable due to one unpaired electron in the p-subshell. Hence, the ${{\text{C}}_{\text{2}}}$ will be the stable form of ${\text{C}}$ as it will form a dichloride molecule (${\text{C}}{{\text{l}}_{\text{2}}}$).
Correct Answer: Option (ii)
16. The molecular formula of the compound formed from B and C will be:
(i) $ BC$
(ii) ${B_2}C$
(iii) $B{C_2}$
(iv) $B{C_3}$
Ans: The electronic configuration of ${\text{B}}$given as\[1{s^2}{\text{ }}2{s^2}{\text{ }}2{p^6}{\text{ }}3{s^2}{\text{ }}3{p^3}\] which represents phosphorous atom. The electronic configuration of ${\text{C}}$ is given as\[1{s^2}{\text{ }}2{s^2}{\text{ }}2{p^6}{\text{ }}3{s^2}{\text{ }}3{p^5}\]which represents chlorine molecule.
So, the molecular formula representing ${\text{B}}$ and${\text{C}}$ will be phosphorous trichloride (${\text{PC}}{{\text{l}}_{\text{3}}}$) which corresponds to ${\text{B}}{{\text{C}}_{\text{3}}}$.
Correct Answer: Option (iv)
17. The bond between B and C will be:
(i) Ionic
(ii) Covalent
(iii) Hydrogen
(iv) Coordinate
Ans: The element ${\text{B}}$ represents the electronic configuration of phosphorus atom and element ${\text{C}}$ represents the electronic configuration of chlorine atom.
Both phosphorus and chlorine are non-metals hence the bonding forming between them will be a covalent bond.
Correct Answer: Option (ii)
18. Which of the following order of energies of molecular orbitals of ${{\text{N}}_{\text{2}}}$ is correct?
(i) $(\Pi 2{p_y}) < (\sigma 2{p_z}) < ({\Pi ^*}2{p_x})\gg ({\Pi ^*}2{p_y})$
(ii) $(\Pi 2{p_y}) > (\sigma 2{p_z}) > ({\Pi ^*}2{p_x})\gg ({\Pi ^*}2{p_y})$
(iii) $(\Pi 2{p_y}) < (\sigma 2{p_z}) > ({\Pi ^*}2{p_x})\gg ({\Pi ^*}2{p_y})$
(iii) $(\Pi 2{p_y}) > (\sigma 2{p_z}) < ({\Pi ^*}2{p_x})\gg ({\Pi ^*}2{p_y})$
Ans: In the molecules of boron (${{\text{B}}_{\text{2}}}$), carbon (${{\text{C}}_{\text{2}}}$) and nitrogen (${{\text{N}}_{\text{2}}}$) the energy of $\sigma 2{p_z}$ molecular orbital is greater than the energy of $\Pi 2{p_x}$ and $\Pi 2{p_y}$ molecular orbital.
So, the correct order of energies of molecular orbital of ${{\text{N}}_{\text{2}}}$ is $(\Pi 2{p_y}) < (\sigma 2{p_z}) < ({\Pi ^*}2{p_x}) \approx ({\Pi ^*}2{p_y})$.
19. Which of the following statements is not correct from the view point of molecular orbital theory?
(i) $B{e_2}$ is not a stable molecule.
(ii) $H{e_2}$ is not stable but $H{e_2}^ + $ expected to exist.
(iii) Bond strength of ${N_2}$ is maximum amongst the homonuclear diatomic molecules belonging to the second period.
(iv) The order of energies of molecular orbitals in ${{\text{N}}_{\text{2}}}$ molecule is $\sigma 2s < {\sigma ^*}2s < \sigma 2{p_z} < (\Pi 2{p_x} = \Pi 2{p_y}) < ({\Pi ^*}2{p_x} = {\Pi ^*}2{p_y}) < {\sigma ^*}2{p_z}$
Ans: Rules for stability of a molecule:
(a) A molecule which has a zero bond order never exists and a molecule having a non-zero bond order either exists or is expected to exist.
(b) If the value of bond order is high then the bond strength will also be higher. Electrons present in the bonding molecular orbital are known as the bonding molecular orbital (${N_b}$) and the electrons present in the anti-bonding molecular orbital are known as anti-bonding electrons (${N_a}$) . The half of the difference of bonding and anti-bonding electrons is known as the bond order.
The bond order of ${\text{B}}{{\text{e}}_{\text{2}}}$:$B{e_2} = \sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2}$
The bonding and anti-bonding electrons are both $4$. Then the bond order (BO) will be:
${\text{BO}} = \dfrac{1}{2}(4 - 4)$
${\text{BO}} = 0$
Bond order of ${\text{B}}{{\text{e}}_{\text{2}}}$ is zero. So, it does not exist
Correct Answer: Option (iv)
20. Which of the following options represents the correct bond order:
(i) \[{O_2}^ - > {O_2} > {O_2}^ + \]
(ii) \[{O_2}^ - < {O_2} < {O_2}^ + \]
(iii) ${O_2}^ - > {O_2} < {O_2}^ + $
(iv) ${O_2}^ - < {O_2} > {O_2}^ + $
Ans: The electronic configurations and the bond orders of the given species are given below:
Bond order of ${{\text{O}}_2}^ -$:
Electronic configuration:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2(\Pi 2{p_x}^2 = \Pi 2{p_y}^2)({\Pi ^*}2{p_x}^1 = {\Pi ^*}2{p_y}^1)$
Bond order = $\dfrac{1}{2}({N_b} - {N_a})$
${\text{BO}} = \dfrac{1}{2}(10 - 7) = \dfrac{3}{2} = 1.5$
Bond order of ${{\text{O}}_2}^ + $:
Electronic configuration:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2(\Pi 2{p_x}^2 = \Pi 2{p_y}^2)({\Pi ^*}2{p_x}^1 = {\Pi ^*}2{p_y}^0)$
${\text{BO}} = \dfrac{1}{2}(10 - 5) = \dfrac{5}{2} = 2.5$
Bond order of ${{\text{O}}_2}$:
Electronic configuration:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2(\Pi 2{p_x}^2 = \Pi 2{p_y}^2)({\Pi ^*}2{p_x}^1 = {\Pi ^*}2{p_y}^1)$
${\text{BO}} = \dfrac{{10 - 6}}{2} = \dfrac{4}{2} = 2$ So, the correct order of bond order is \[{{\text{O}}_2}^ - < {{\text{O}}_2} < {{\text{O}}_2}^ + \].
Correct Answer: Option (ii)
21:The electronic configuration of the outer most shell of the most electronegative element is
(i) $2{s^2},2{p^5}$
(ii) $3{s^2},3{p^5}$
(iii) $4{s^2},4{p^5}$
(iv) $5{s^2},5{p^5}$
Ans: The most electronegative element in the periodic table is fluorine having an atomic number $9$. The electronic configuration of fluorine is $ 1{s^2},2{s^2},2{p^5}$ . Hence, the electronic configuration of the outermost shell of fluorine is $2{s^2},2{p^5}$.
Correct Answer: Option (i)
22:Amongst the following elements whose electronic configurations are given below, the one having the highest ionisation enthalpy is
(i) $[Ne]3{s^2}3{p^1}$
(ii) $[Ne]3{s^2}3{p^3}$
(iii) $[Ne]3{s^2}3{p^2}$
(iv) $[Ar]3{d^{10}}4{s^2}4{p^3}$
Ans: Aluminium has $[{\text{Ne}}]3{s^2}3{p^1}$ electronic configuration. Phosphorus has $[{\text{Ne}}]3{s^2}3{p^3}$. The electronic configuration of silicon is $[{\text{Ne}}]3{s^2}3{p^2}$ and the electronic configuration of arsenic is $[{\text{Ar}}]3{d^{10}}4{s^2}4{p^3}$.
Ionisation energy increases as the atomic number in a period increases and decreases on moving down the group. It is also known that the ionisation enthalpy of group $15$ elements is more than group $16$ elements as group $15$ has half-filled p-subshells which gives extra stability.
Thus, the electronic configuration having highest ionisation enthalpy is $[{\text{Ne}}]3{s^2}3{p^3}$.
Correct Answer: Option (ii)
MULTIPLE CHOICE QUESTIONS (TYPE-II):
In the following questions two or more options may be correct.
23. Which of the following have identical bond order?
(i) \[C{N^ - }\]
(ii) $ N{O^ + }$
(iii) ${O_2}^ -$
(iv) ${O_2}^{2 - }$
Ans: Bond order depends on the electronic configuration of the molecular species, and the electronic configuration depends on the number of electrons a particular atom is contributing in its molecular structure.
The\[{\text{C}}{{\text{N}}^ - }\] species has a total of $14$ electrons; $6$ electrons from carbon atom, $7$ electrons from nitrogen atom and $1$ electron for the negative charge.
The ${\text{N}}{{\text{O}}^ + }$ species has a total of $14$ electrons; $8$ from oxygen atoms,$7$ from nitrogen atom and $1$ electron will be subtracted due to the positive charge on the species.
The ${{\text{O}}_2}^ -$ species has a total of $17$ electrons;$8$ from each oxygen atom and $1$ for the negative charge on the species.
The ${{\text{O}}_2}^{2 - }$ species has a total of $18$ electrons; $8$ from each oxygen atom and $2$ for the negative charge on the species.
Hence, the molecular species/ion having the same bond order will be \[{\text{C}}{{\text{N}}^ - }\] and ${\text{N}}{{\text{O}}^ + }$.
Correct Answer: Option (i) and option (ii)
24.Which of the following attain the linear structure:
(i) $ BeC{l_2}$
(ii) $ NC{O^ + }$
(iii) $ N{O_2}$
(iv) $ C{S_2}$
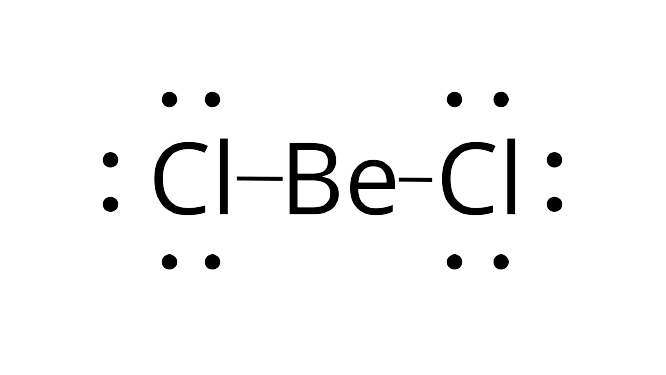
Ans: Beryllium chloride ${\text{BeC}}{{\text{l}}_{\text{2}}}$ does have the presence of any lone pair of electrons in its structure. So, it will attain a linear structure.
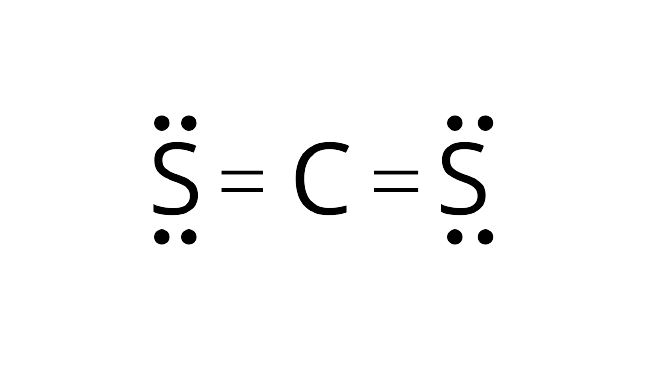
Carbon disulphide ${\text{C}}{{\text{S}}_{\text{2}}}$ does not have any lone pair of electron on carbon atom. Thus, it will attain a linear structure.
The structures of ${\text{N}}{{\text{O}}_{\text{2}}}$ and ${\text{NC}}{{\text{O}}^{\text{ + }}}$ has a lone pair of electron on nitrogen atom thus it will form a bent shape rather than a linear shape.
Correct Answer: Option (i) and option (iv)
25.$CO$ is isoelectronic with
(i) $ N{O^ + }$
(ii) ${N_2}$
(iii) $ SnC{l_2}$
(iv) $ N{O_2}^ -$
Ans: The species having the same number of electrons are known as isoelectronic species.
The number of electrons in ${\text{CO}}$species is $14$;$8$ electrons from oxygen atom and $6$ electrons from carbon atom.
The number of electrons in ${\text{N}}{{\text{O}}^ + }$ is $14$;$8$ electrons from oxygen atom,$7$ electrons from nitrogen atom and $1$ electron will be subtracted due to the positive charge on the species.
The number of electrons in ${{\text{N}}_{\text{2}}}$ is $14$ ;$7$ from each nitrogen atom.
The number of electrons in ${\text{SnC}}{{\text{l}}_{\text{2}}}$ is $84$ ;$50$ from the tin atom and $17$ from each chlorine atom.
The number of electrons in ${\text{N}}{{\text{O}}_2}^ -$ is $24$ ;$7$ from nitrogen atom,$8$ from each oxygen atom and $1$ from the negative charge on the species.
Hence, ${\text{N}}{{\text{O}}^ + }$ and ${{\text{N}}_{\text{2}}}$ are the isoelectronic species of ${\text{CO}}$.
Correct Answer: Option (i) and option (ii)
26. Which of the following species have the same shape?
(i) $ C{O_2}$
(ii) $ CC{l_4}$
(iii) ${O_3}$
(iv) $ N{O_2}^ -$
Ans:(i) The number of valence electrons in ${\text{C}}{{\text{O}}_{\text{2}}}$ is $14$. The oxygen atom attached to the carbon atom forms a double bond. Therefore, the hybridization of the structure is $s{p^2}$. Thus, carbon dioxide is linear in shape.
(ii) The number of valence electrons in ${\text{CC}}{{\text{l}}_{\text{4}}}$ is $32$. The structure of ${\text{CC}}{{\text{l}}_{\text{4}}}$ is arranged in a tetrahedral manner. The chlorine atoms are arranged such that the hybridization is $s{p^3}$.
(iii) The number of valence electrons in ${{\text{O}}_{\text{3}}}$ is $24$. The hybridization of the molecule is $s{p^2}$ and due to the presence of lone pair of electrons on the central oxygen atom and due to the effect of resonance, ozone molecule has a bent shape.
(iv) The number of valence electrons in ${\text{N}}{{\text{O}}_{\text{2}}}^ -$ is $24$. The hybridization of the molecule is $s{p^2}$ and due to the presence of lone pair on the nitrogen atom it attains a bent shape.
Correct Answer: Option (iii) and option (iv)
27.Which of the following statements are correct about$C{O_3}^{2 - }$?
(i) The hybridization of central atom is $s{p^3}$.
(ii) Its resonance structure has one $C - O$single bond and two $C = O$ double bonds.
(iii) The average formal charge on each oxygen atom is $0.67$ units.
(iv) All $C - O$ bond lengths are equal.
Ans: (i) The hybridization of the central carbon atom in ${\text{C}}{{\text{O}}_3}^{2 - }$ is $s{p^2}$ as the carbon is bonded with three oxygen atoms. So, the given statement is incorrect.
(ii) The resonance structure of ${\text{C}}{{\text{O}}_3}^{2 - }$ has one ${\text{C}} = {\text{O}}$ double bonds and two ${\text{C}} - {\text{O}}$ single bonds. So, the statement is incorrect.
(iii) The net charge on three oxygen atoms is $ - 2$ , hence the average formal charge on each oxygen atom is $0.67$ units. So, the given statement is correct.
(iv) As it can be seen from the resonating structures that the structures of the bonds are not fixed. Hence, all the ${\text{C}} - {\text{O}}$ bond lengths are equal. So, the given statement is correct.
Correct Answer: Option (iii) and option (iv)
28. Diamagnetic species are those which contain no unpaired electrons. Which among the following are diamagnetic?
(i) ${N_2}$
(ii) ${N_2}^{2 - }$
(iii) ${O_2}$
(iv) ${O_2}^{2 - }$
Ans: (i) ${{\text{N}}_2}$ will have $14$ electrons in its structure. So, its electronic configuration will be: $\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\Pi 2{p_x}^2,\Pi 2{p_y}^2,\sigma 2{p_z}^2$. According to the molecular electronic configuration of ${{\text{N}}_2}$ it does not contain any unpaired electrons, it will be diamagnetic in nature.
(ii) ${{\text{N}}_2}^{2 - }$ will have $16$ electrons in its structure. So, its molecular electronic configuration will be: $\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\Pi 2{p_x}^2,\Pi 2{p_y}^2,\sigma 2{p_z}^2,{\Pi ^*}2{p_x}^1,{\Pi ^*}2{p_y}^1$. According to the molecular electronic configuration of ${{\text{N}}_2}^{2 - }$, it has two unpaired electrons in ${\Pi ^*}2{p_x}^1,{\Pi ^*}2{p_y}^1$ sub-shell. Hence, it will be paramagnetic in nature.
(iii) ${{\text{O}}_2}$ will have $16$ electrons in its structure. So, its molecular electronic configuration will be: $\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_y}^2,\Pi 2{p_x}^2,{\Pi ^*}2{p_y}^1,{\Pi ^*}2{p_x}^1$ . According to the molecular electronic configuration of ${{\text{O}}_2}$, it has two unpaired electrons in ${\Pi ^*}2{p_x}^1,{\Pi ^*}2{p_y}^1$ sub-shell. Hence, it will be paramagnetic in nature.
(iv) ${{\text{O}}_2}^{2 - }$ will have $18$ electrons in its structure. So, its molecular electronic configuration will be: $\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_y}^2,\Pi 2{p_x}^2,{\Pi ^*}2{p_y}^2,{\Pi ^*}2{p_x}^2$. According to the molecular electronic configuration of ${{\text{O}}_2}^{2 - }$ it does not contain any unpaired electrons, it will be diamagnetic in nature.
Correct Answer: Option (i) and option (iv)
29. Species having same bond order are :
(i) ${N_2}$
(ii) ${N_2}^ -$
(iii) ${F_2}^ + $
(iv) ${O_2}^ -$
Ans:The formula of bond order is:
${\text{BO}} = \dfrac{1}{2}[{N_b} - {N_a}]$
Number of bonding and antibonding electrons can be found from the molecular electronic configuration of the species.
(i)Total number of electrons in ${{\text{N}}_{\text{2}}}$ is $14$ . So, its molecular electronic configuration will be $\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\Pi 2{p_x}^2,\Pi 2{p_y}^2,\sigma 2{p_z}^2$.
The bond order will be:
${\text{BO}} = \dfrac{1}{2}(10 - 4) = 3$
(ii)The total number of electrons in ${{\text{N}}_2}^ -$ is $15$ . So, its molecular electronic configuration will be: $\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\Pi 2{p_x}^2,\Pi 2{p_y}^2,\sigma 2{p_z}^2,{\Pi ^*}2{p_x}^1$
The bond order will be:
$BO = \dfrac{1}{2}(10 - 5) = 2.5$
(iii)The total electrons in ${{\text{F}}_{\text{2}}}^{\text{ + }}$ is $17$ . So, its electronic configuration will be: $\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_y}^2,\Pi 2{p_x}^2,{\Pi ^*}2{p_y}^2,{\Pi ^*}2{p_x}^1$
The bond order will be:
${\text{BO}} = \dfrac{1}{2}(10 - 7) = 1.5$
(iv)The total electrons in${{\text{O}}_{\text{2}}}^ -$ is $17$ So, its electronic configuration will be: $\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_y}^2,\Pi 2{p_x}^2,{\Pi ^*}2{p_y}^2,{\Pi ^*}2{p_x}^1$
The bond order will be:
${\text{BO}} = \dfrac{1}{2}(10 - 7) = 1.5$
Correct Answer: Option (iii) and option (iv)
30.Which of the following statements are not correct?
(i) $NaCl$ being an ionic compound is a good conductor of electricity in the solid state.
(ii) In canonical structures there is a difference in the arrangement of atoms.
(iii) Hybrid orbitals form stronger bonds than pure orbitals.
(iv) VSEPR Theory can explain the square planar geometry of $Xe{F_4}$.
Ans: (i) ${\text{NaCl}}$ being an ionic compound is a good conductor of electricity in the molten state. It is not a bad conductor of electricity in solid state. So, the statement mentioned in the question is not correct. Hence, this is the correct answer.
(ii) There is no difference in the arrangement of atoms in the canonical structures. The difference is in the arrangement of electron pairs in the canonical structures. So, the statement mentioned in the question is not correct. Hence, this is the correct answer.
(iii) The hybrid orbitals have the same energy and shape and hence they are more effective in the formation of stable bonds than the pure atomic orbitals. So, the statement given is correct. Hence, this is not the correct answer.
(iv) $Xe{F_4}$acquires a square planar geometry, this can very well be explained by the VSEPR theory. The hybridisation of the central xenon atom will be $s{p^3}{d^2}$ due to the presence of two lone pairs of electrons on the central xenon atom. So, the statement given is correct. Hence, this is not the correct answer.
Correct Answer: Option (i) and option (ii)
SHORT ANSWER TYPE:
31.Explain the non linear shape of ${H_2}S$ and non planar shape of $PC{l_3}$using valence shell electron pair repulsion theory.
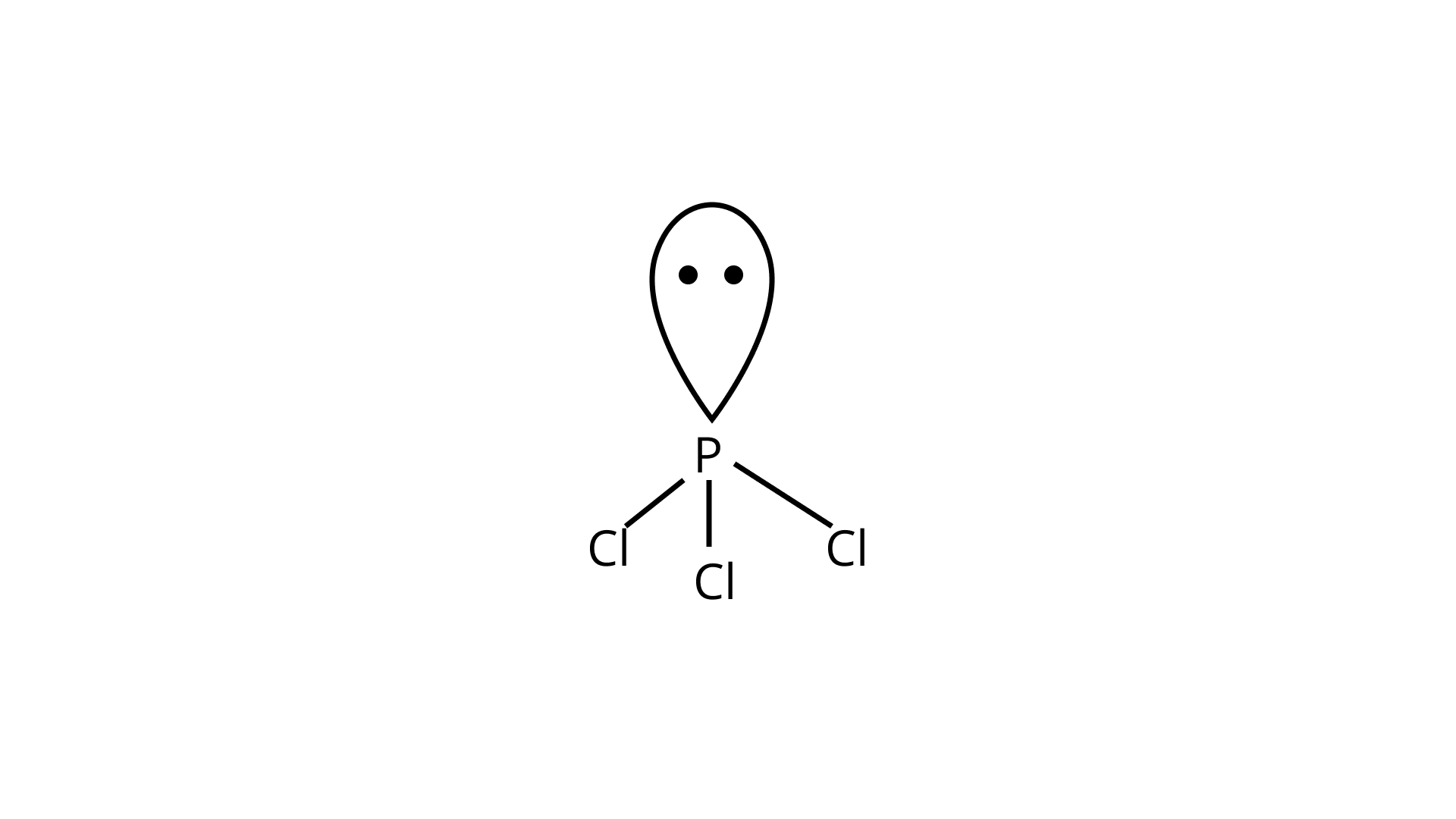
Ans: In the Lewis structure of ${\text{PC}}{{\text{l}}_{\text{3}}}$, the phosphorus atom is surrounded by three bond pairs (chlorine atoms) and one lone pair. These four electron pairs are arranged in a tetrahedral geometry around the central phosphorus atom. Due to the presence of lone pair of electron on the phosphorus atom, ${\text{PC}}{{\text{l}}_{\text{3}}}$ will have a distorted tetrahedral geometry. Thus, it will form a pyramidal shape and is non-linear in structure.
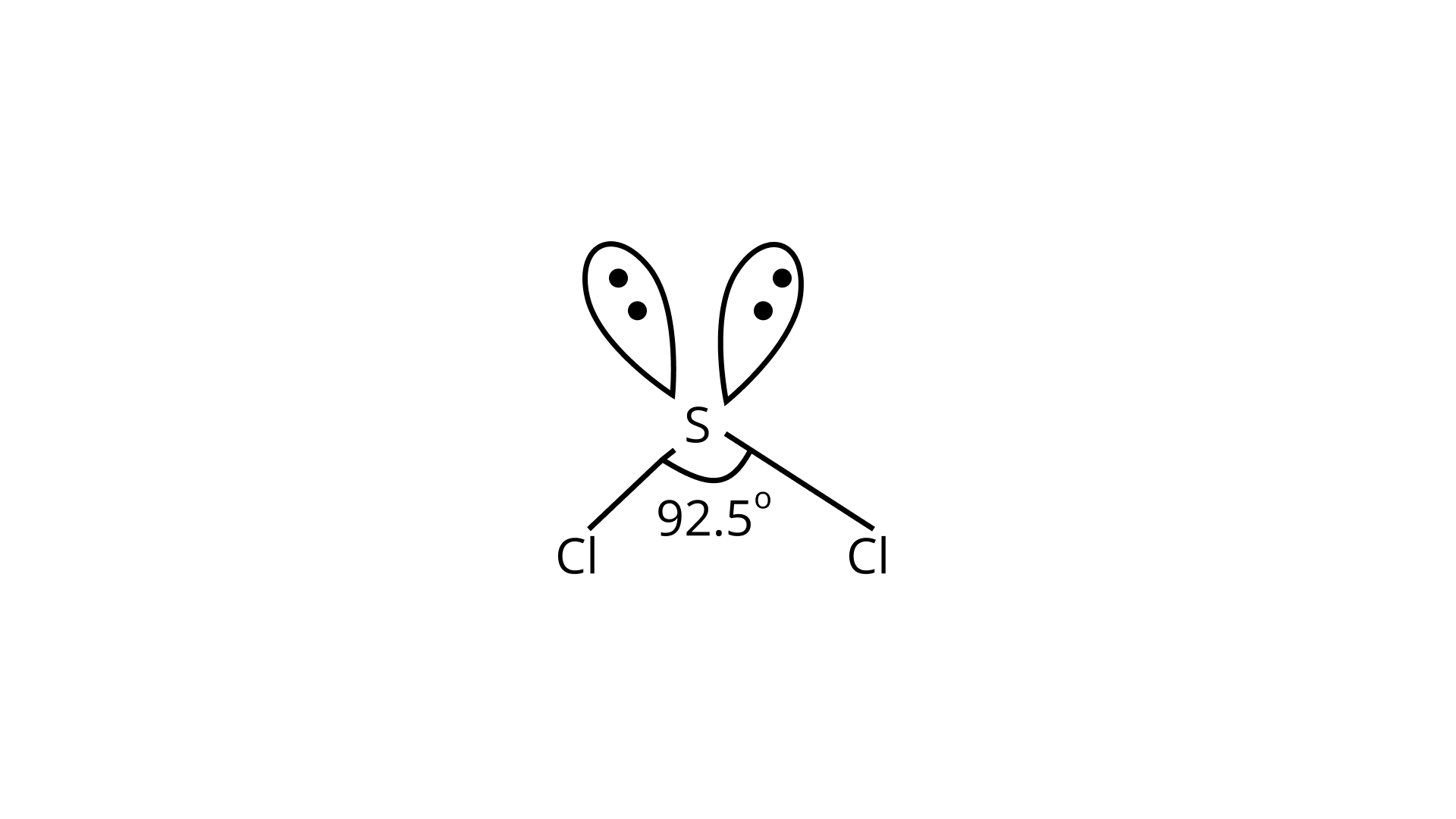
In ${{\text{H}}_{\text{2}}}{\text{S}}$, the central sulphur atom is surrounded by two bond pairs and two lone pairs of electrons. It can be said that these four electron pairs are arranged in a tetrahedral geometry. Due to the presence of two lone pairs on the central sulphur atom, the lone pair-bond pair repulsion happens and due to this the molecule ${{\text{H}}_{\text{2}}}{\text{S}}$ has a V-shaped geometry and is non-linear in structure.
32.Using molecular orbital theory, compare the bond energy and magnetic character of ${O_2}^ + $ and ${O_2}^ -$ species.
Ans:The electronic configurations of ${{\text{O}}_2}^ + $ and ${{\text{O}}_2}^ -$ according to molecular orbital theory is:
${{\text{O}}_2}^ + :$$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_y}^2,\Pi 2{p_x}^2,{\Pi ^*}2{p_x}^1$
${{\text{O}}_2}^ - :$$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_y}^2,\Pi 2{p_x}^2,{\Pi ^*}2{p_x}^2,{\Pi ^*}2{p_y}^1$
The bond order of ${{\text{O}}_2}^ + :$
${\text{BO}} = \dfrac{1}{2}[{N_b} - {N_a}]$
${\text{BO}} = \dfrac{1}{2}[10 - 5] = \dfrac{5}{2}$
${\text{BO}} = 2.5$
The bond order of ${{\text{O}}_2}^ - :$
${\text{BO}} = \dfrac{1}{2}[{N_b} - {N_a}]$
${\text{BO}} = \ddfrac{1}{2}[10 - 7] = \dfrac{3}{2}$
${\text{BO}} = 1.5$
As the bond order of ${{\text{O}}_2}^ + $ is higher, it is more stable than ${{\text{O}}_2}^ -$, because higher the bond order more stable is the bond. Both the molecular species have the presence of unpaired electrons. So, they both are paramagnetic in nature.
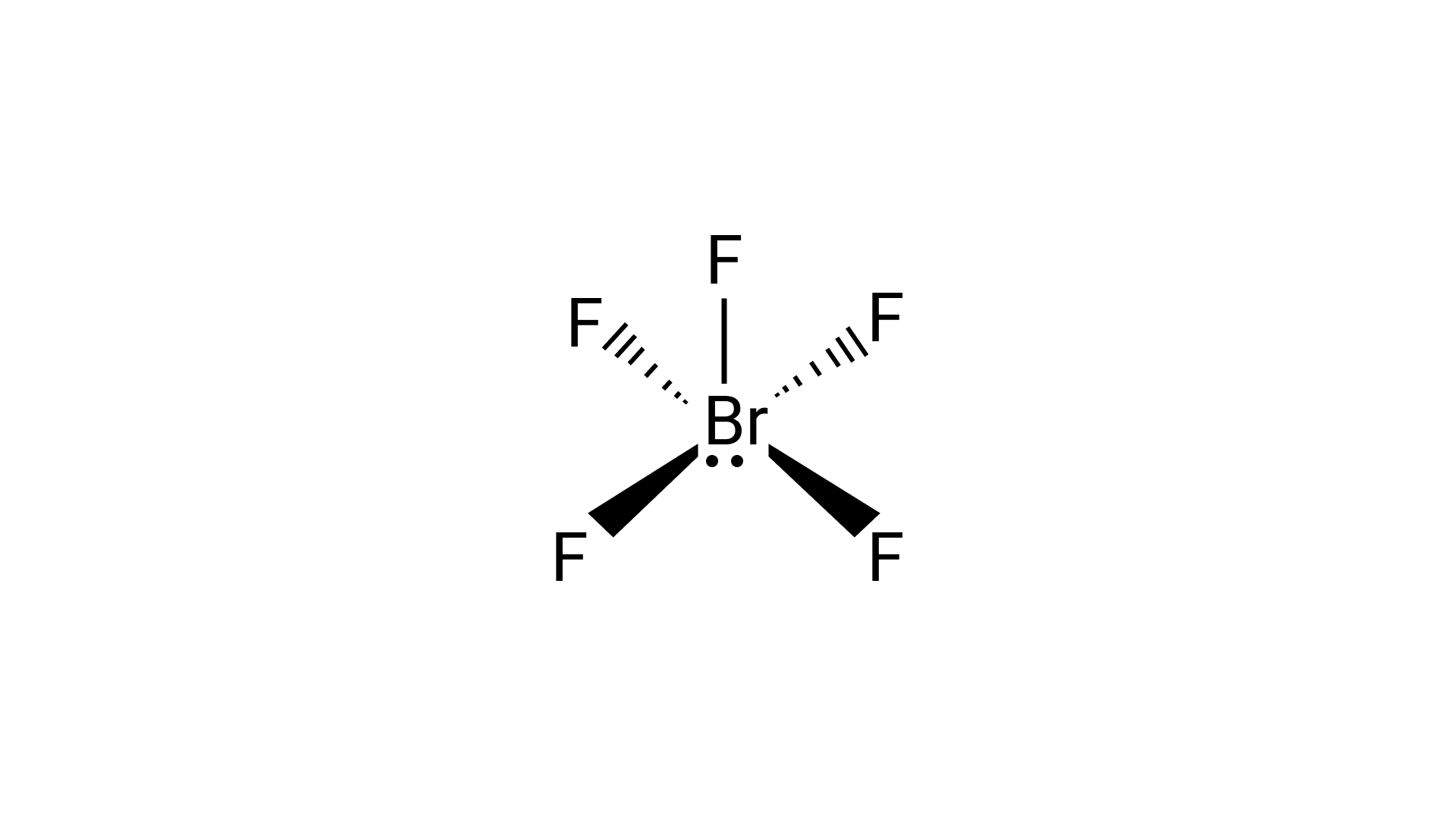
33.Explain the shape of $Br{F_5}$.
Ans: In ${\text{Br}}{{\text{F}}_{\text{5}}}$the central bromine atom has $7$ valence electrons. It makes five bonds with the fluorine atom and one lone pair of electron is left. Due to the lone pair-bond pair repulsions, ${\text{Br}}{{\text{F}}_{\text{5}}}$makes a structure of square pyramidal geometry. Due to the distortion of fluorine ions, each fluorine ion makes an angle of ${90^ \circ }$.
The square pyramidal shape of ${\text{Br}}{{\text{F}}_{\text{5}}}$ is:
34.Structures of molecules of two compounds are given below :
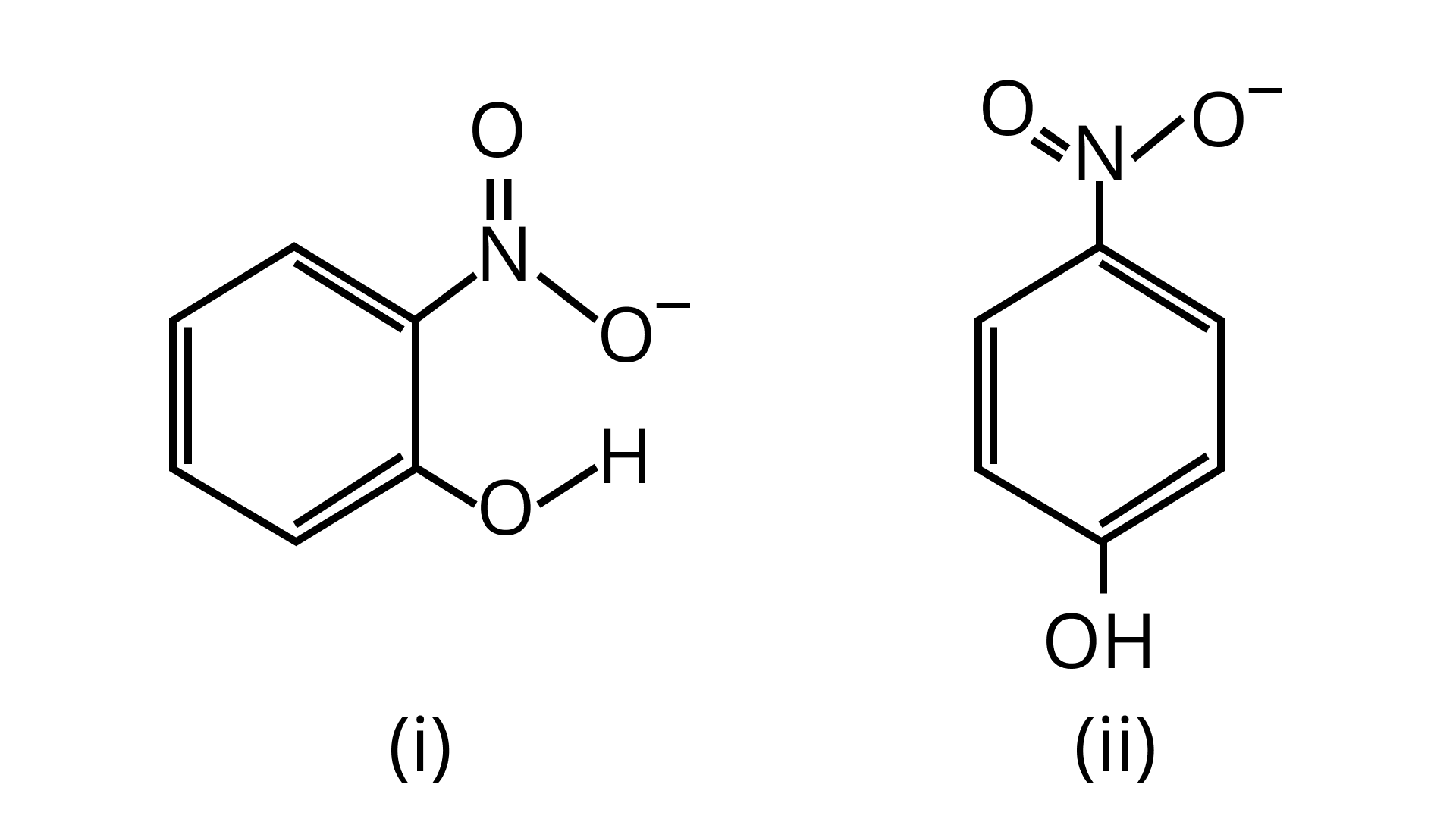
(a)Which of the two compounds will have intermolecular hydrogen bonding and which compound is expected to show intramolecular hydrogen bonding?
Ans: The intramolecular hydrogen bonding is shown by compound (I) and the intermolecular hydrogen bonding is shown by compound (II). In compound (I) the ${\text{N}}{{\text{O}}_{\text{2}}}$ and ${\text{OH}}$ group are close together in comparison to that in compound (II). So, that is why compound (I) shows intramolecular hydrogen bonding.
The intermolecular hydrogen bonding in compound (II) is shown as:
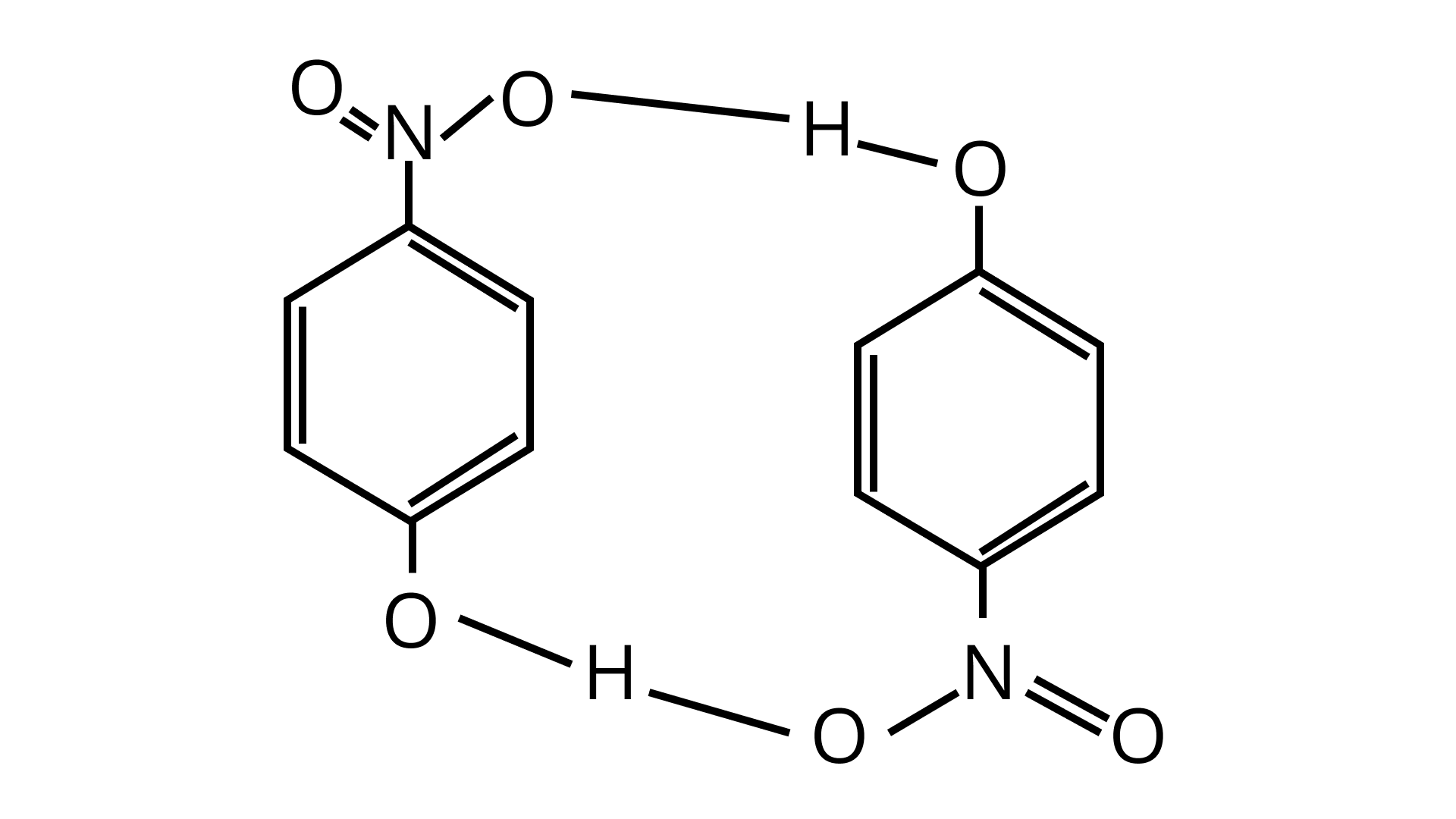
(b)The melting point of a compound depends on, among other things, the extent of hydrogen bonding. On this basis explain which of the above two compounds will show higher melting point.
Ans: Due to the formation of intermolecular hydrogen bonds, compound (II) will have a higher melting point. Thus, due to hydrogen bond formation more molecules are joined together.
(c)Solubility of compounds in water depends on power to form hydrogen bonds with water. Which of the above compounds will form hydrogen bond with water easily and be more soluble in it?
Ans: Compound (I) will be less soluble in water due to intramolecular hydrogen bonding; it will not be able to form hydrogen bonds with water. Whereas, compound (II) can form hydrogen bonds with water more easily and is thus more soluble in water.
35. Why does type of overlap given in the following figure not result in bond formation?
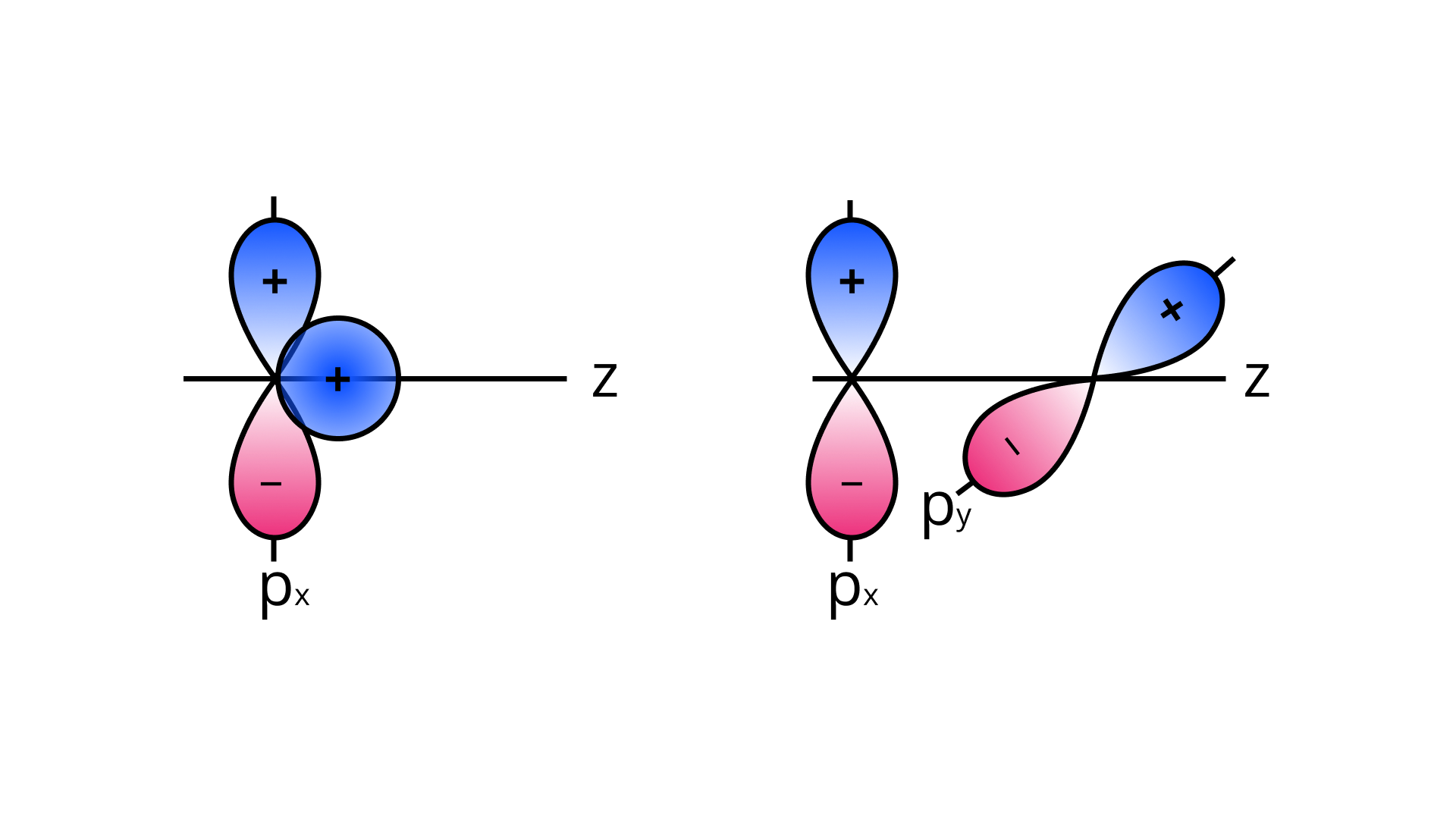
Ans: In the first figure given above, the area which is under $+ +$ overlapping is equal to the area under $+-$ overlap. Both the overlaps cancel out with each other as they are oppositely charged. Due to cancelling out of the overlaps the net overlap will be zero.
In the second figure given above, both the p-orbitals are perpendicular to each other. Due to the ${p_x},{p_y}$ orbitals being perpendicular with each other, no overlap will be possible.
36. Explain why $PC{l_5}$ is trigonal bipyramidal whereas${\text{I}}{{\text{F}}_{\text{5}}}$ is square pyramidal.
Ans: In the structure of ${\text{PC}}{{\text{l}}_{\text{5}}}$, phosphorus atoms forms five bonds with the chloride ion. Phosphorus has $5$ valence shell electrons and it uses them to form five bonds with chlorine by sharing its electron from $3s$ and $3d$ orbitals. Hence, the hybridisation of ${\text{PC}}{{\text{l}}_{\text{5}}}$ will be $s{p^3}d$. Due to the absence of lone pair of electrons on the phosphorus atom, the chloride ions are arranged in such a way that three bond pairs are attached at equatorial position and two bonds are attached at axial position. Thus making a trigonal bipyramidal geometry.
In the structure of ${\text{I}}{{\text{F}}_{\text{5}}}$, there are $7$ electrons in the valence shell of iodine. Out of the $7$ electrons, iodine shares $5$ to make bonds with the five fluoride ions. Two electrons are left which forms one lone pair on the iodine atom. Due to the presence of a lone pair on an iodine atom, the bond pairs are arranged in such a manner that they form an angle of ${90^ \circ }$. Thus, forming a square pyramidal shape. Thus, the hybridisation of ${\text{I}}{{\text{F}}_{\text{5}}}$ is $s{p^3}{d^2}$.
37.In both water and dimethyl ether, oxygen atom is central atom, and has the same hybridisation, yet they have different bond angles. Which one has greater bond angle? Give reason.
Ans: Although the hybridisation of the central atom oxygen in both the molecules is $s{p^3}$ but dimethyl ether will have a higher bond angle than water molecule.
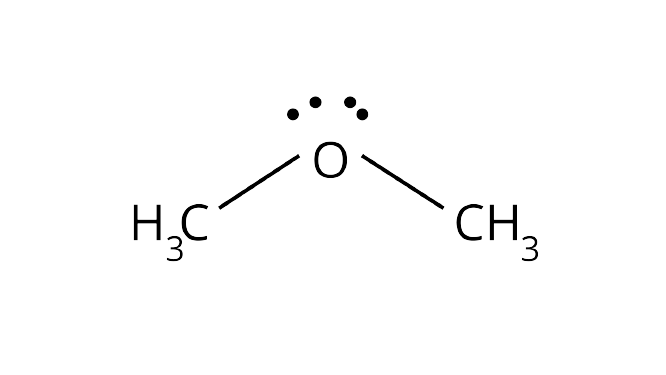
Due to the presence of two bulky methyl groups in dimethyl ether, the repulsive forces will be greater in them than the two hydrogens in water molecules. In dimethyl ether the $ - {\text{C}}{{\text{H}}_{\text{3}}}$ is a group attached to three hydrogen atom through $\sigma $ bonds. Thus, the${\text{C}} - {\text{H}}$ bond pairs increase the electron density on the carbon atom which results in lone pair-bond pair repulsions. Due to this lone pair-bond pair repulsions, the bond angle of dimethyl ether is greater than that of water molecules.
38. Write Lewis structure of the following compounds and show formal charge on each atom.
$HN{O_3},{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} N{O_2},{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {H_2}S{O_4}$
Ans: The Lewis structures of the compounds are:
Lewis structure for nitric acid:
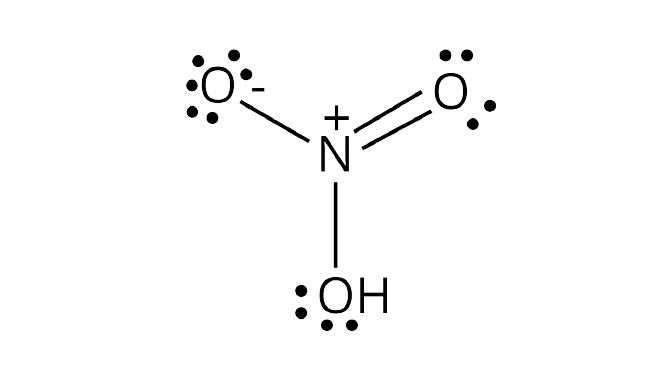
Lewis structure of nitrogen dioxide:
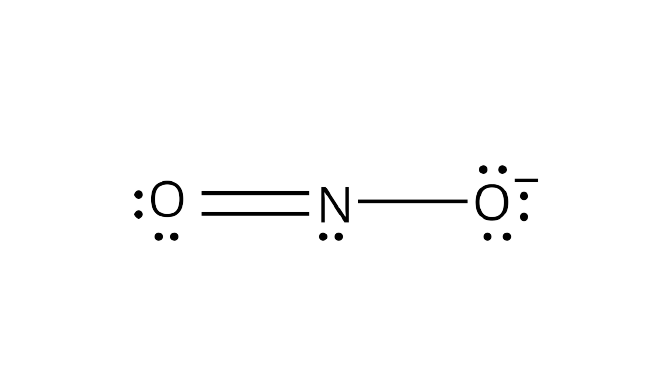
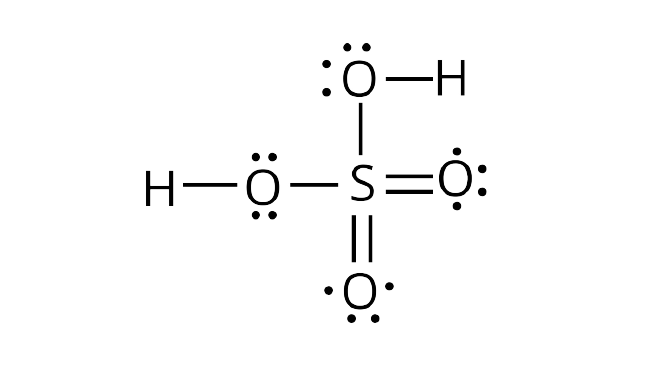
Lewis structure of sulphuric acid:
39. The energy of $\sigma 2{p_z}$ molecular orbital is greater than$\Pi 2{p_x}$ and$\Pi 2{p_y}$ molecular orbitals in nitrogen molecule. Write the complete sequence of energy levels in the increasing order of energy in the molecule. Compare the relative stability and the magnetic behaviour of the following species :
${N_{2,}} {N_2}^ + , {N_2}^ - , {N_2}^{2 + }$
Ans: The general molecular energy levels for nitrogen atom is:
$\sigma 1s < {\sigma ^*}1s < \sigma 2s < {\sigma ^*}2s < \Pi 2px = \Pi 2py < \sigma 2pz$
${{\text{N}}_{\text{2}}}$ molecule has $14$ electrons in its molecular structure. Its electronic configuration will be:
$\sigma 1{s^2} < {\sigma ^*}1{s^2} < \sigma 2{s^2} < {\sigma ^*}2{s^2} < \Pi 2p{x^2} = \Pi 2p{y^2} < \sigma 2p{z^2}$
${{\text{N}}_{\text{2}}}^{\text{ + }}$has $13$ electrons in its molecular structure. Its electronic configuration will be:
$\sigma 1{s^2} < {\sigma ^*}1{s^2} < \sigma 2{s^2} < {\sigma ^*}2{s^2} < \Pi 2p{x^2} = \Pi 2p{y^2} < \sigma 2p{z^1}$
${{\text{N}}_2}^ -$ has $15$ electrons in its molecular structure. Its electronic configuration will be:
$\sigma 1{s^2} < {\sigma ^*}1{s^2} < \sigma 2{s^2} < {\sigma ^*}2{s^2} < \Pi 2p{x^2} = \Pi 2p{y^2} < \sigma 2p{z^2} < {\Pi ^*}2p{x^1}$
${N_2}^{2 + }$has $12$ electrons in its molecular structure. Its electronic configuration will be:
$\sigma 1{s^2} < {\sigma ^*}1{s^2} < \sigma 2{s^2} < {\sigma ^*}2{s^2} < \Pi 2p{x^2} = \Pi 2p{y^2}$
The formula for finding the bond order (B. O) for any molecular species is:
${\text{BO}} = \dfrac{1}{2}[{N_b} - {N_a}]$
Hence, the bond orders of the given molecular species are:
${{\text{N}}_2} = \dfrac{{10 - 4}}{2} = 3$
${{\text{N}}_2}^ + = \dfrac{{9 - 4}}{2} = 2.5$
${{\text{N}}_2}^ - = \dfrac{{10 - 5}}{2} = 2.5$
${{\text{N}}_2}^{2 + } = \dfrac{{8 - 4}}{2} = 2$
The order for the stability of the given molecular species thus will be:
${{\text{N}}_2} > {{\text{N}}_2}^ - > {{\text{N}}_2}^ + > {{\text{N}}_2}^{2 + }$
40.What is the effect of the following processes on the bond order in${N_2}$ and${O_2}$ ?
(i)
Ans: The effect of the following process on the bond order: ${{\text{N}}_2} \to {{\text{N}}_2}^ + + {{\text{e}}^ - }$ :
The electronic configuration of ${{\text{N}}_2}$ is:
$\sigma 1{s^2} < {\sigma ^*}1{s^2} < \sigma 2{s^2} < {\sigma ^*}2{s^2} < \Pi 2p{x^2} = \Pi 2p{y^2} < \sigma 2p{z^2}$
So, its bond order will be $3$ .
The electronic configuration of ${{\text{N}}_2}^ + $ is:
$\sigma 1{s^2} < {\sigma ^*}1{s^2} < \sigma 2{s^2} < {\sigma ^*}2{s^2} < \Pi 2p{x^2} = \Pi 2p{y^2} < \sigma 2p{z^1}$
Its bond order will be $2.5$
Hence the bond order decreases in this reaction ${{\text{N}}_2} \to {{\text{N}}_2}^ + + {{\text{e}}^ - }$
Ans: The effect of the following process on the bond order:
${{\text{O}}_2} \to {{\text{O}}_2}^ + + {{\text{e}}^ - }$:
The electronic configuration of ${{\text{O}}_2}$ is:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_y}^2,\Pi 2{p_x}^2,{\Pi ^*}2{p_x}^2$
Its bond order will be $2$
The electronic configuration of ${{\text{O}}_2}^ + $ will be:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_y}^2,\Pi 2{p_x}^2,{\Pi ^*}2{p_x}^1$
Its bond order will be $2.5$ .
Hence, the bond order in this reaction ${{\text{O}}_2} \to {{\text{O}}_2}^ + + {{\text{e}}^ - }$ increases.
41.Give reasons for the following :
(i) Covalent bonds are directional bonds while ionic bonds are non-directional.
Ans: Due to the electrostatic forces between the two opposite charges, ionic bonds are non-directional. The bonding direction does not matter as the electrostatic field of an ion is non-directional. Whereas, the formation of covalent bonds happens with the overlap of the atomic orbitals. The direction of the bonds is given by the direction of overlapping.
(ii)Water molecule has bent structure whereas carbon dioxide molecule is linear.
Ans: The hybridization of the oxygen atom in water molecules is $s{p^3}$ due to the presence of two lone pairs of electrons on the oxygen atom. Tetrahedral geometry is acquired by these four $s{p^3}$ hybridized orbitals with two bonds of hydrogen atoms and two lone pairs of electrons. Due to the presence of lone pairs of electron the bond angle gets reduced as there is a repulsion between the LP-LP and thus the molecule acquires a bent shape.
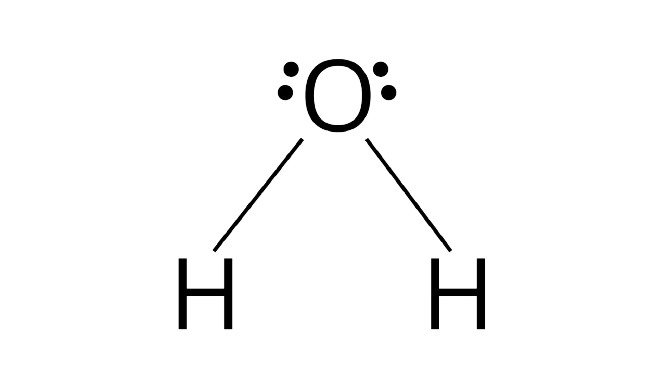
The hybridization of carbon atoms in the carbon dioxide molecule is $sp$. The two $sp$are arranged in such a way that it makes an angle of ${180^ \circ }$. That is why carbon dioxide has a linear shape.
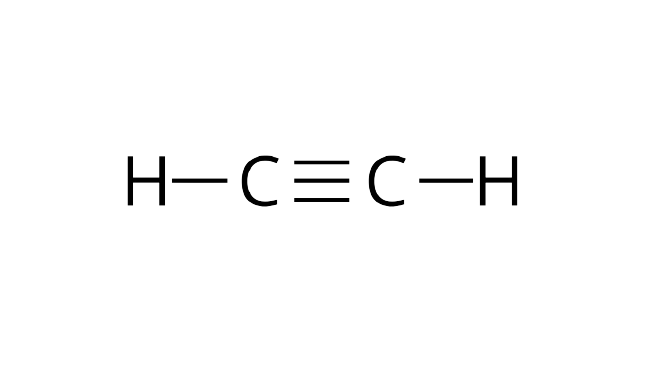
(iii) Ethyne molecule is linear.
Ans: Both the carbon atoms in ethyne molecules are $sp$ hybridized. Ethyne has two unhybridized$2{p_x},2{p_y}$ orbitals. The two$sp$hybrid orbitals of both the carbon atoms are arranged such that they form an angle of ${180^ \circ }$with each other. That is why ethyne molecules are linear in shape.
42. What is an ionic bond? With two suitable examples explain the difference between an ionic and a covalent bond?
Ans: Ionic bonds are such bonds in which complete transfer of electrons from one atom to another atom. Due to complete transfer positive and negative ions are formed in this bond. The ions in this bond are held together by electrostatic force of attraction. The formation of calcium fluoride ${\text{Ca}}{{\text{F}}_{\text{2}}}$ leads to the formation of an ionic bond.
$Ca \to C{a^{2 + }} + 2{e^ - }$ The electronic configuration of $Ca = [Ar]{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} 4{s^2}$ and $C{a^{2 + }} = [Ar]$
$F + {e^ - } \to {F^ - }$ The electronic configuration of $F = [He]{\kern 1pt} {\kern 1pt} 2{s^2}{\kern 1pt} {\kern 1pt} 2{p^5}$ and ${F^ - } = [He]{\kern 1pt} {\kern 1pt} 2{s^2}{\kern 1pt} {\kern 1pt} 2{p^6}$
Thus, $C{a^{2 + }} + 2{F^ - } \to Ca{F_2}$
A covalent bond is formed due to mutual sharing of electrons between two atoms of a non-metal. This mutual sharing of electrons between the two non-metal species leads to the formation of a covalent bond. The making of ${\text{C}}{{\text{l}}_{\text{2}}}$ leads to a formation of a covalent bond between two chloride ions.
43.Arrange the following bonds in order of increasing ionic character giving reason
\[N---H, F---H, C---H and O---H\]
Ans: The ionic character in a molecular species is decided by the electronegativity difference between the two bonded atoms. Greater the electronegativity difference between two bonded pairs, the greater will be the ionic character.
The electronegativity difference of the given species is:
C - F = (2.5 - 2.1) = 0.4
N - H = (3.0 - 2.1) = 0.9
O - H = (3.5 - 2.1) = 1.4
F - H = (4.0 - 2.1) = 1.9
According to the above given difference in the electronegativities, the order of increasing ionic character is:
${\text{C - H}} < {\text{N - H}} < {\text{O - H}} < {\text{F - H}}$
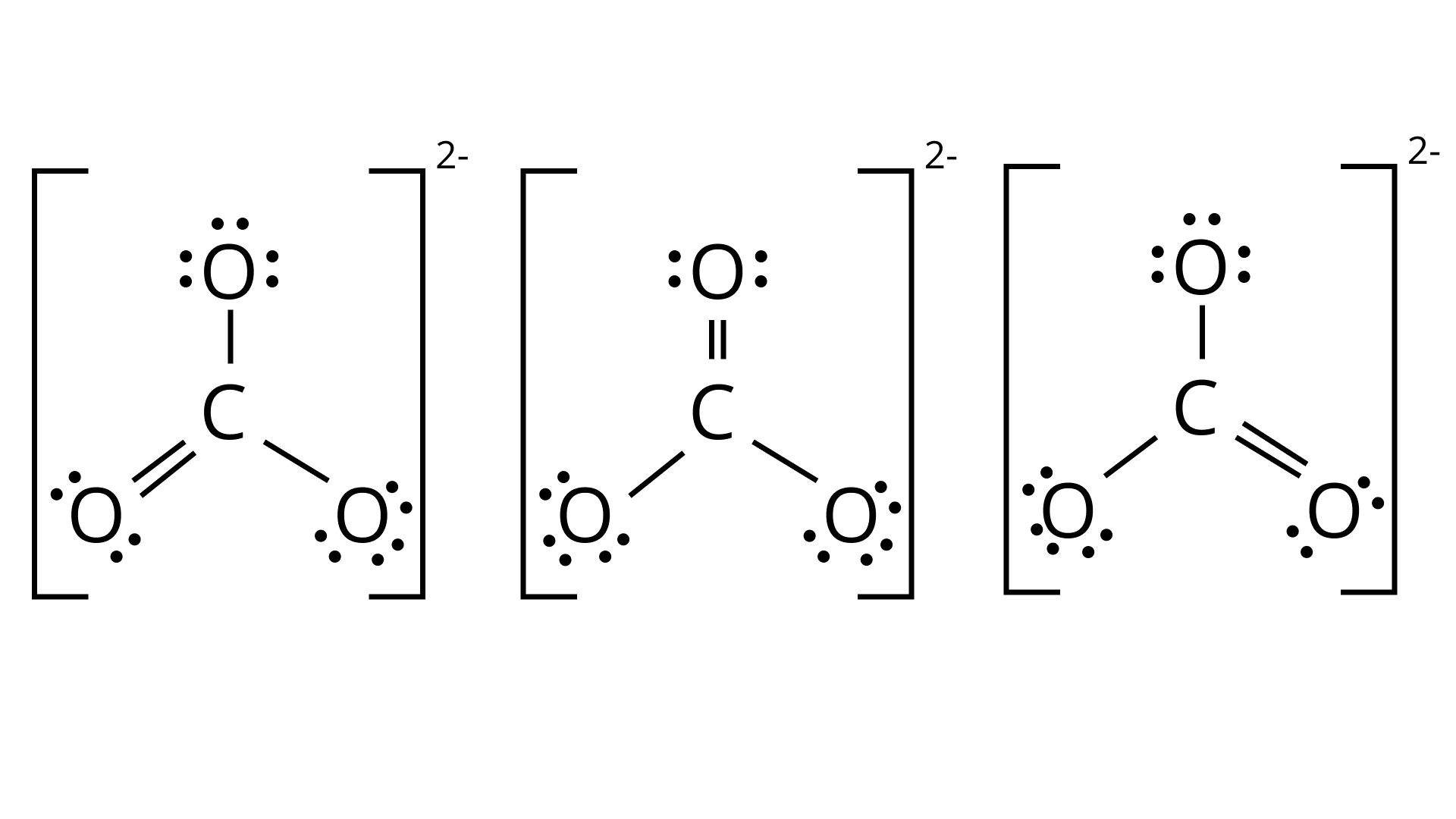
44. Explain why $C{O_3}^{2 - }$ ion cannot be represented by a single Lewis structure. How can it be best represented?
Ans: The carbonate ion ${\text{C}}{{\text{O}}_3}^{2 - }$ can be best represented by its resonating structures. The carbonate ion cannot be represented by a single Lewis structure because the three carbon oxygen bond lengths are the same. This cannot be shown by a single Lewis structure. For showing the similar lengths of all the carbon to oxygen bonds three hybrid structures are constructed which are in resonance with each other.
45.Predict the hybridisation of each carbon in the molecule of organic compound given below. Also indicate the total number of sigma and pi bonds in this molecule.
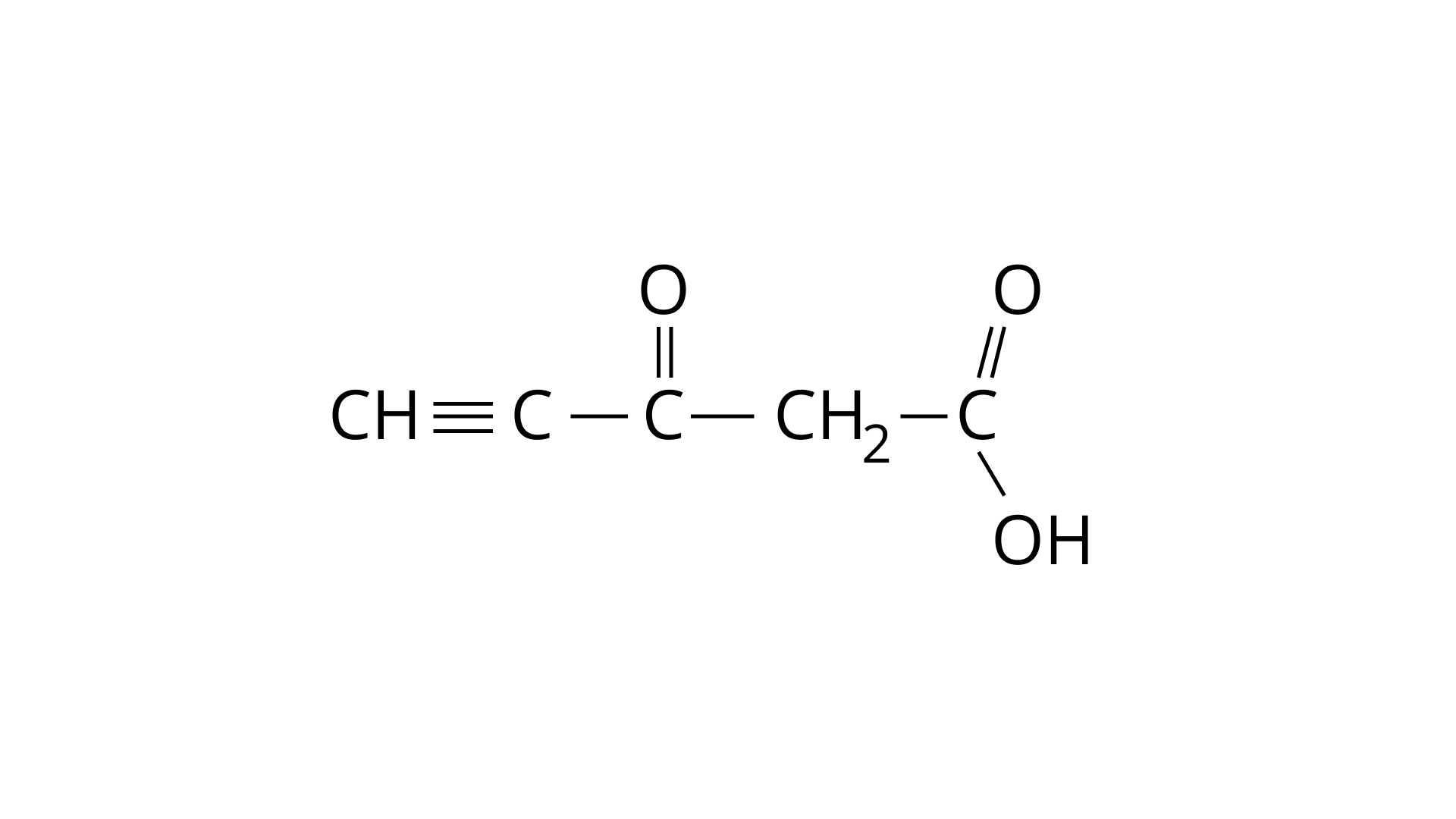
Ans: In the above structure, two carbon atoms which are linked through a triple bond are $sp$ hybridized. Two carbonyl ($ - {\text{C}} = {\text{O}}$) carbon atoms are $s{p^2}$ hybridized. The $ - {\text{C}}{{\text{H}}_{\text{2}}}$ carbon atom is $s{p^3}$ hybridized.
From the above given molecule it is clear that there are $11 - \sigma $ bonds and $4 - \Pi $ bonds.
46.Group the following as linear and non-linear molecules :
\[{H_2}O, HOCl, BeC{l_2}, C{l_2}O\]
Ans:The molecules containing oxygen atom have the presence of two lone pair of electrons and due to lone pair-lone pair repulsions the molecules ${{\text{H}}_{\text{2}}}{\text{O}},{\text{HOCl}},{\text{C}}{{\text{l}}_{\text{2}}}{\text{O}}$ will have a non-linear shape.
The structure of ${\text{BeC}}{{\text{l}}_{\text{2}}}$ as the central beryllium atom will not have any lone pair of electrons.
47.Elements $X$,$Y$ and $Z$ have $4$,$5$ and$7$valence electrons respectively. (i) Write the molecular formula of the compounds formed by these elements individually with hydrogen. (ii) Which of these compounds will have the highest dipole moment?
Ans: (i)According to the electronic configuration, a total of $8$ electrons must be present in the valence shell of an element. Element ${\text{X}}$has $4$ valence electrons, so it will share the remaining $4$ electrons for the formation of the bond, the molecular formula will be ${\text{X}}{{\text{H}}_{\text{4}}}$. The element ${\text{Y}}$ has $5$ valence shell electrons, so it will form $3$ bonds and the formula will be ${\text{Y}}{{\text{H}}_{\text{3}}}$. The element ${\text{Z}}$ has $7$ valence shell electrons, so it will form one bond with hydrogen and has the molecular formula ${\text{H}} - {\text{Z}}$.
(ii) Elements ${\text{X}}$,${\text{Y}}$ and${\text{Z}}$ having $4$,$5$ and $7$ valence electrons respectively belongs to the second period and group ${14^{{\text{th}}}},{15^{{\text{th}}}}{\text{ and 1}}{{\text{7}}^{{\text{th}}}}$. The electronegativity of the elements increases on moving across the period and the dipole moment is calculated by the difference in the electronegativity of the atoms in a molecular structure. Higher the difference in the electronegativity will be the dipole moment. So, the dipole moment of $H - Z$ will be the highest.
48.Draw the resonating structure of
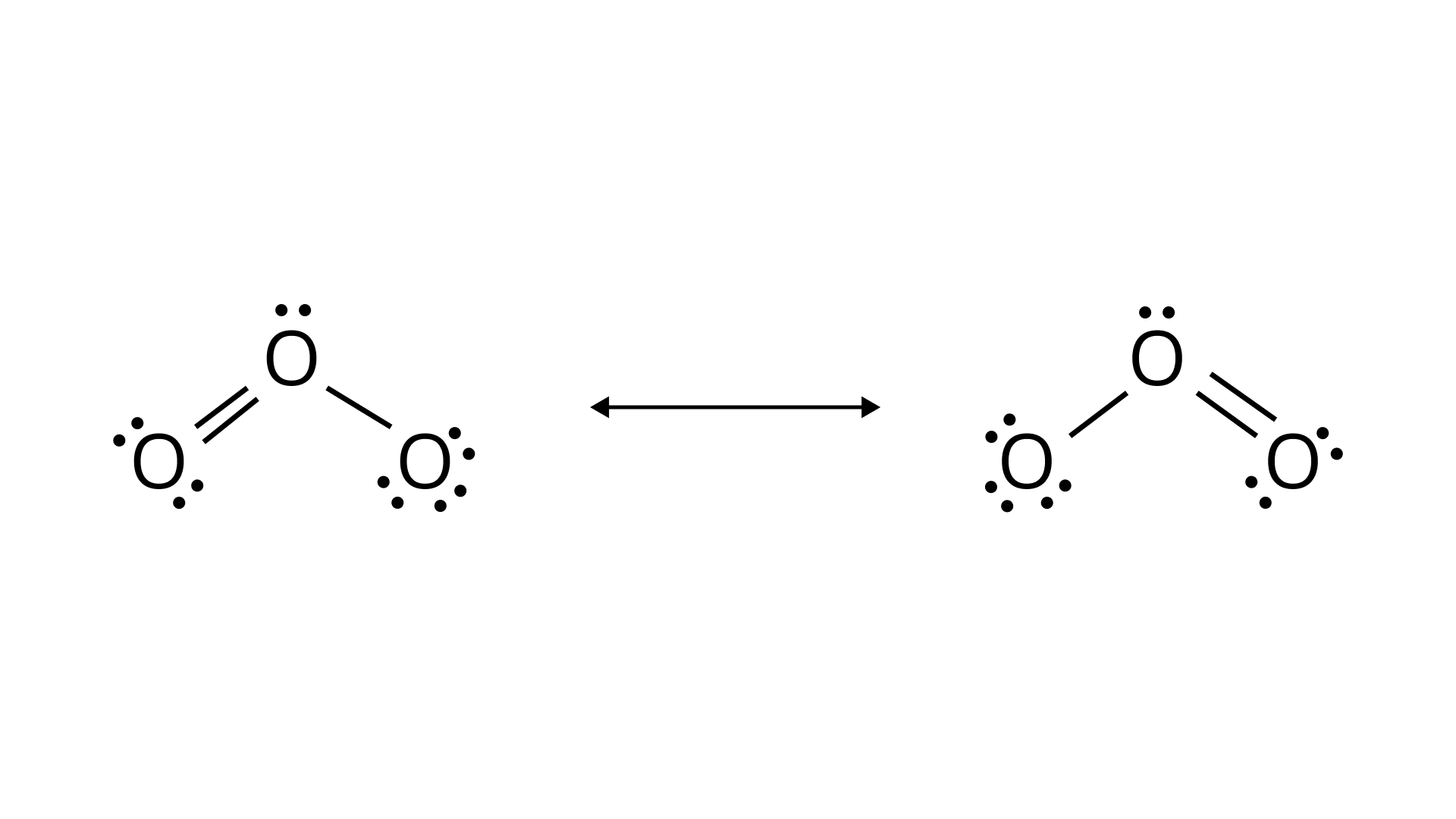
(i)Ozone molecule
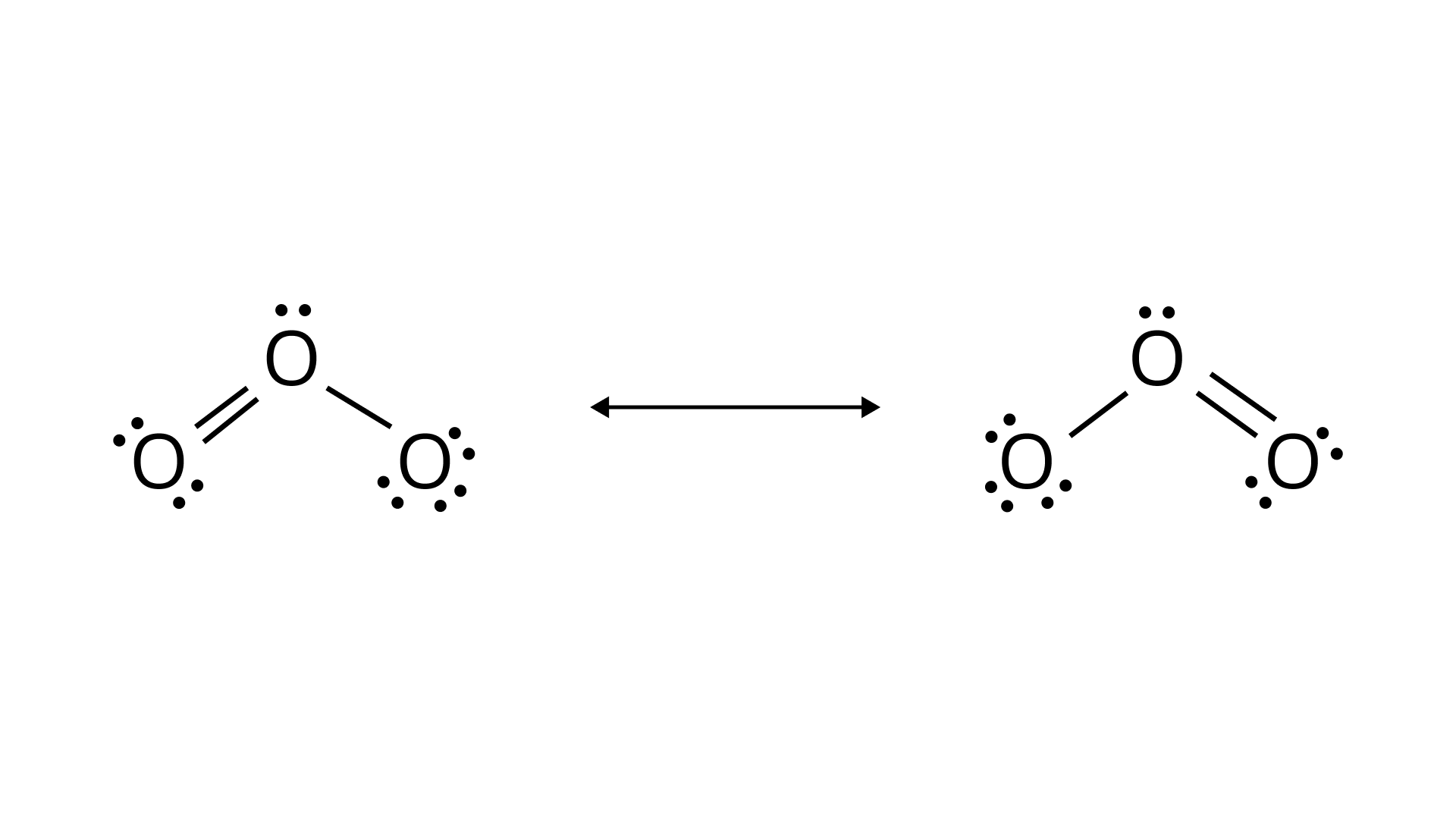
Ans: The resonating structure of the following molecules is given as:
Ozone molecule:
(ii)Nitrate ion
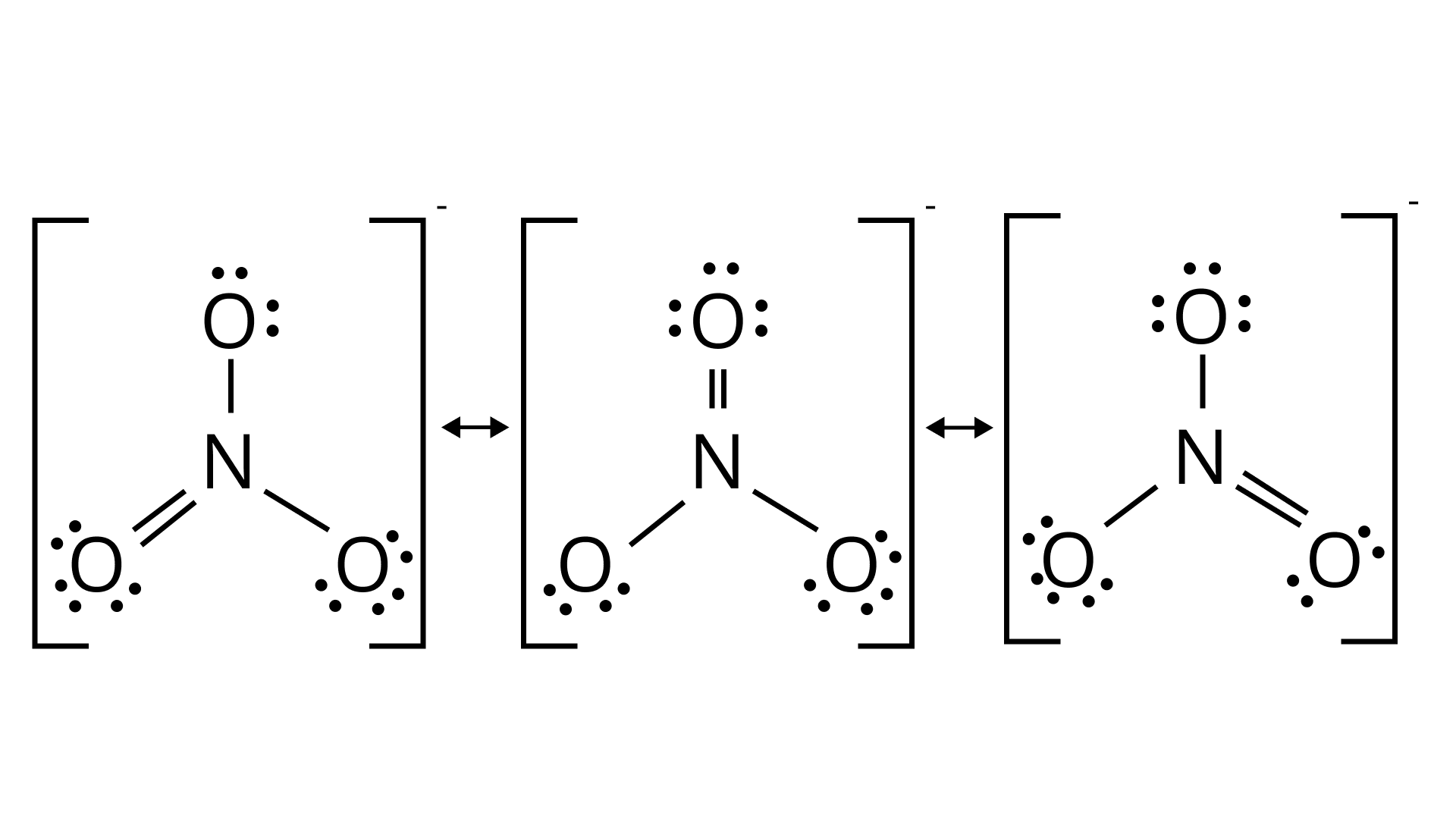
Ans: The resonating structure of the following molecules is given as:
Nitrate ion:
49.Predict the shapes of the following molecules on the basis of hybridisation.
\[BC{l_3}, C{H_4} , C{O_2}, N{H_3}\]
Ans:
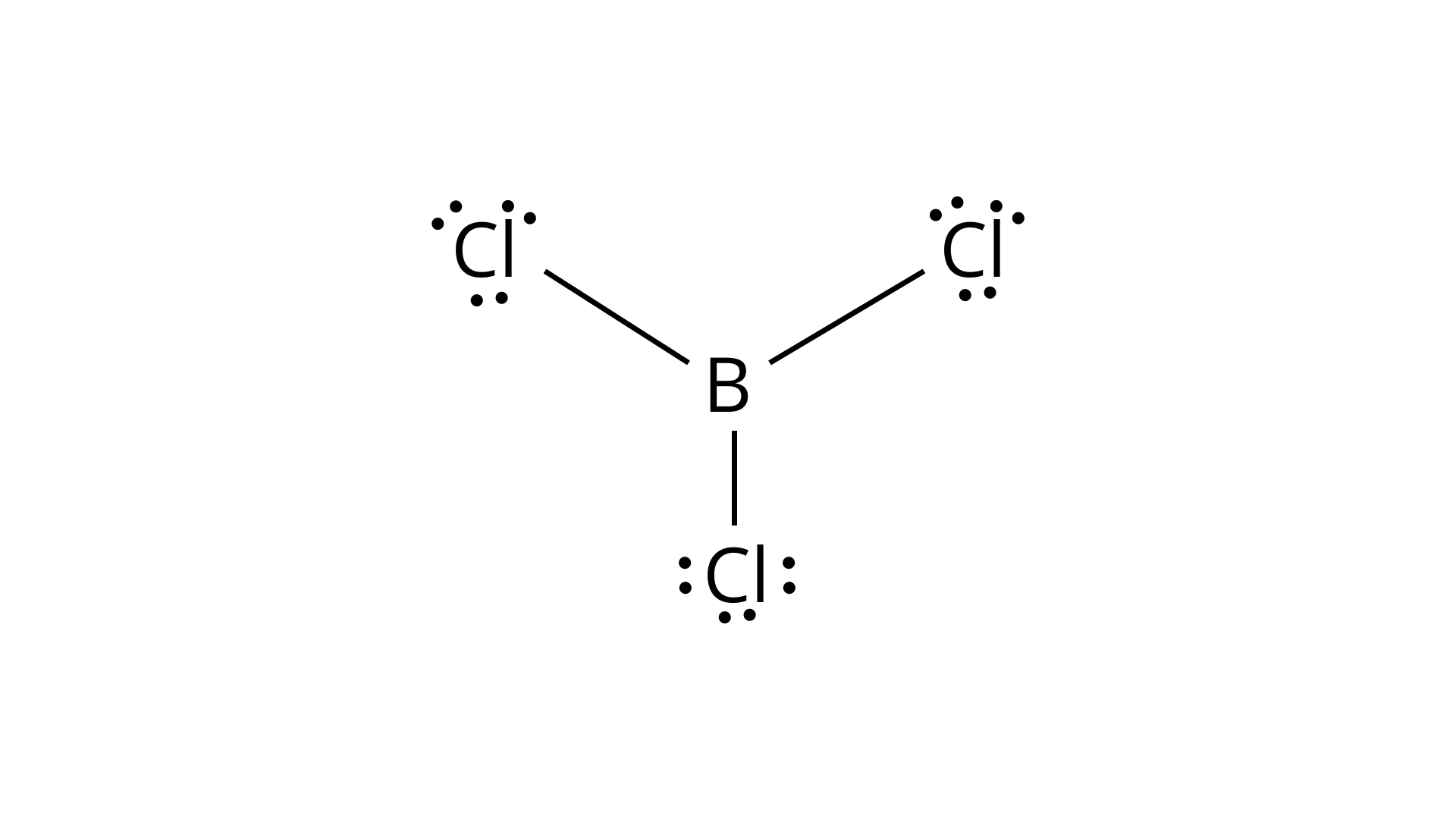
Boron trichloride has $s{p^2}$ hybridization, so according to the above given structure, the shape will be trigonal planar.
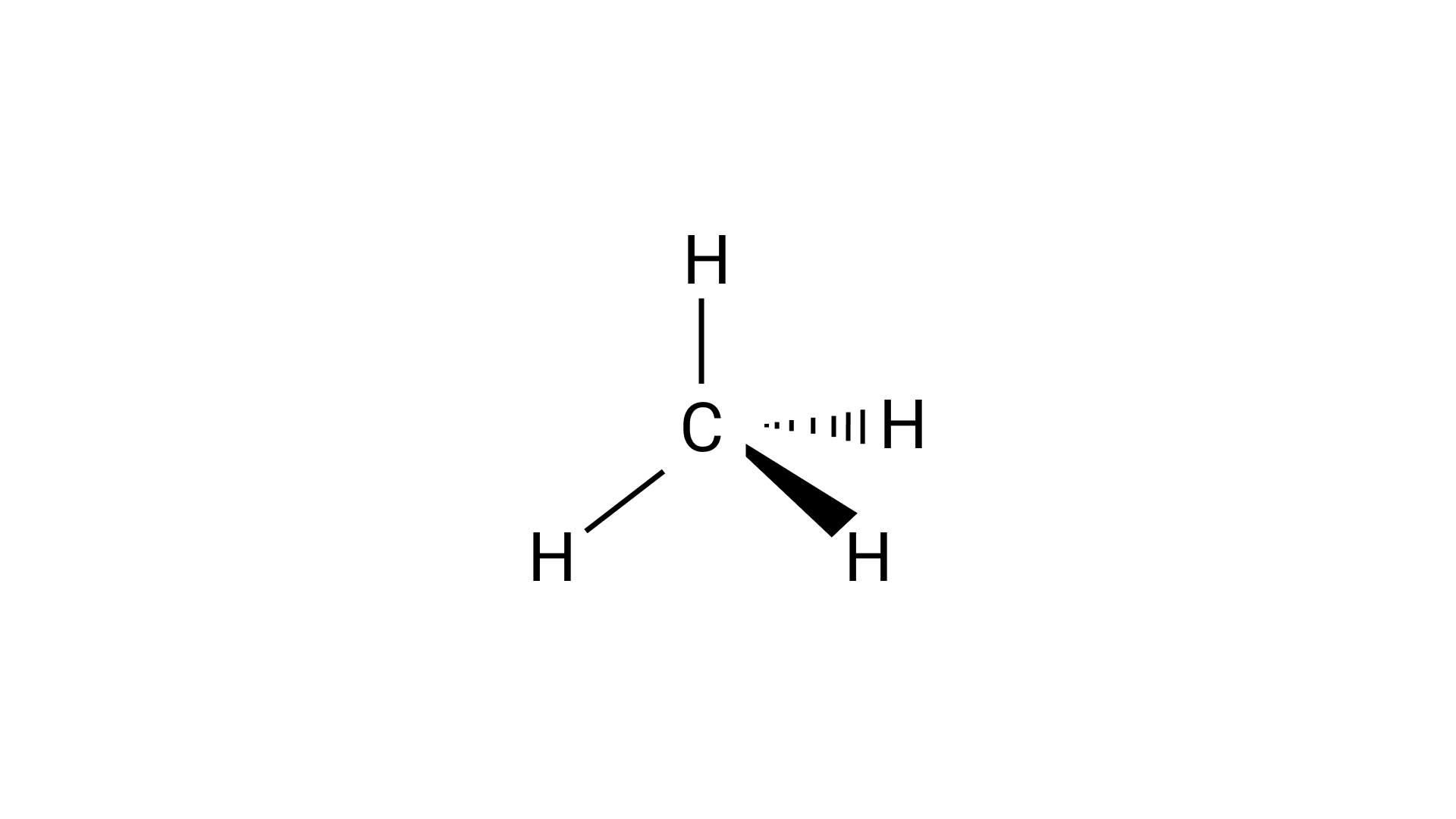
In methane molecules the carbon is $s{p^3}$ hybridized, so according to the above given structure methane will have a tetrahedral shape.
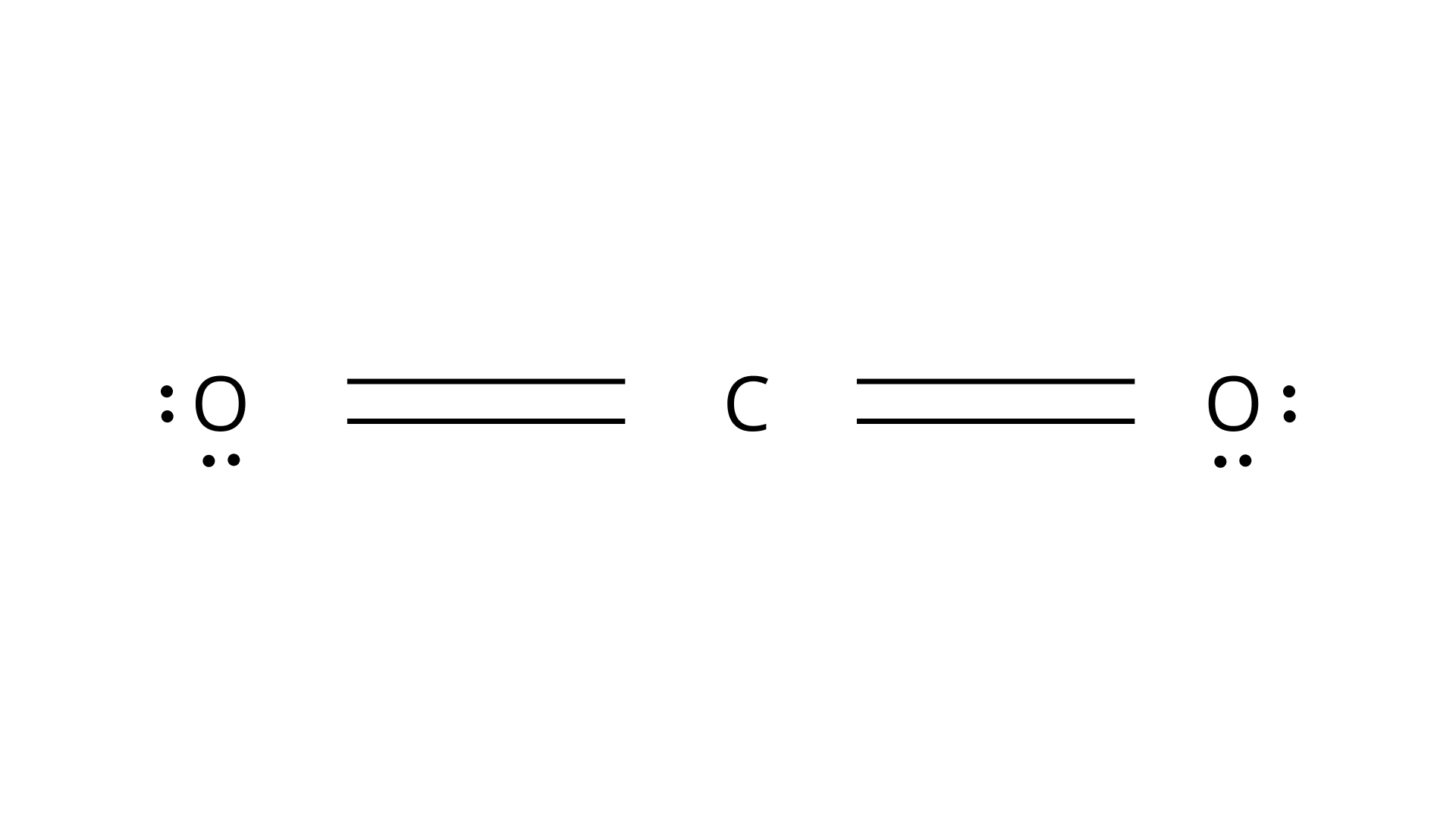
In a carbon dioxide molecule the carbon is $sp$ hybridized, so according to the above structure it will have a linear shape.
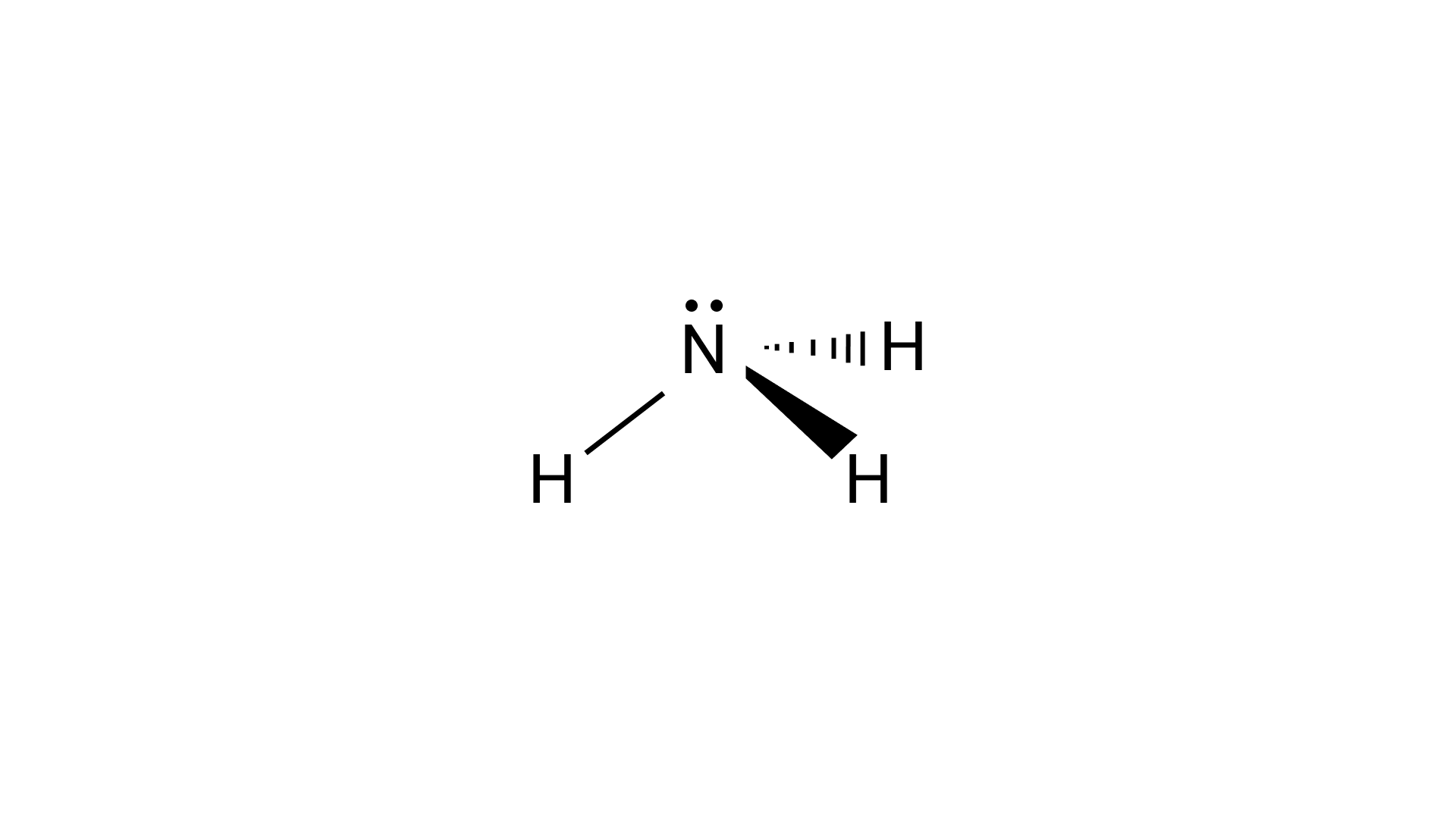
In ammonia molecules, the nitrogen is $s{p^3}$ hybridized and due to the presence of lone pairs of electrons on the nitrogen atom, ammonia will have a pyramidal shape.
50. All the $C - O$ bonds in carbonate ion ($C{O_3}^{2 - }$) are equal in length. Explain.
Ans: All the${\text{C}} - {\text{O}}$ bonds in carbonate ion (${\text{C}}{{\text{O}}_{\text{3}}}^{2 - }$) are equal in length due to the equivalent resonance of all the three carbon-oxygen bonds gets a double bond character atleast once. This type of double bond character happened throughout $3$${\text{C}} - {\text{O}}$skeleton, and hence all the bonds acquired equal bond length.
51.What is meant by the term average bond enthalpy? Why is there difference in bond enthalpy of $O - H$ bond in ethanol (${C_2}{H_5}OH$) and water?
Ans:The average bond enthalpy is defined as the ratio of total bond dissociation enthalpy to the number of bonds broken in the structure.
The identical ${\text{O}} - {\text{H}}$bonds in water molecule does not have the same bond enthalpies. According to the structure of a water molecule, there are two${\text{O}} - {\text{H}}$bonds, but there is a change in the breaking of the first${\text{O}} - {\text{H}}$bond than the second because of the different charge.
Hence in water molecule the average bond enthalpy will be:
The average${\text{O}} - {\text{H}}$bond enthalpy = $\dfrac{{502 + 427}}{2} = 464{\kern 1pt} {\kern 1pt} {\kern 1pt} {\kern 1pt} {\text{kJ}}{\kern 1pt} {\kern 1pt} {\kern 1pt} {\text{mo}}{{\text{l}}^{{\text{ - 1}}}}$
In ethanol${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}$the bond enthalpy of ${\text{O}} - {\text{H}}$ is different because the electronic environment around oxygen atom is different. In ethanol the${\text{O}} - {\text{H}}$ is attached to the carbon atom and in water molecule the${\text{O}} - {\text{H}}$ is attached to the hydrogen atom.
MATCHING TYPE:
52.Match the species in Column${\text{I}}$ with the type of hybrid orbitals in Column${\text{II}}$.
Column${\text{I}}$ | Column${\text{II}}$ | ||
(i) | $S{F_4}$ | (a) | $s{p^3}{d^2}$ |
(ii) | $I{F_5}$ | (b) | ${d^2}s{p^3}$ |
(iii) | $N{O_2}^ + $ | (c) | $s{p^3}d$ |
(iv) | $N{H_4}^ + $ | (d) | $s{p^3}$ |
(e) | $sp$ |
Ans:
Column${\text{I}}$ | Column${\text{II}}$ | ||
(i) | ${\text{S}}{{\text{F}}_{\text{4}}}$ | (c) | $s{p^3}d$ |
(ii) | ${\text{I}}{{\text{F}}_{\text{5}}}$ | (a) | $s{p^3}{d^2}$ |
(iii) | ${\text{N}}{{\text{O}}_{\text{2}}}^{\text{ + }}$ | (e) | $sp$ |
(iv) | ${\text{N}}{{\text{H}}_{\text{4}}}^{\text{ + }}$ | (d) | $s{p^3}$ |
(i) ${\text{S}}{{\text{F}}_{\text{4}}}$ has a total of $34$ valence electrons. Sulphur has $6$ valence electrons and each fluoride has $7$valence electrons. The central atom sulphur will thus share one electron with all the four fluoride ions and thus make four bonds. A lone pair of electrons (two electrons) is left on the sulphur atom. Thus, the sulphur atom is surrounded by five electron pairs, four bond pairs and one lone pair. Having five electron pairs surrounding sulphur, it will have a trigonal bipyramidal geometry with $s{p^3}d$hybridization. Due to the lone pair of electrons, bond pair-lone pair repulsion occurs which causes the bond angle to change. This change in the bond angle causes the distortion in the geometry and changes it into see-saw shape.
(ii) ${\text{I}}{{\text{F}}_{\text{5}}}$has the total of $42$ valence shell electrons,$7$ valence electrons in iodine and $7$ in each of the fluoride ions. The central iodine atom will thus make five sigma bonds with chlorine and a lone pair of electrons will be left on the iodine atom. The six electron pairs thus will have a hybridization of $s{p^3}{d^2}$ and octahedral geometry. Due to the presence of lone pairs of electrons on the iodine, lone pair-bond pair repulsion occurs and the structure forms a distorted octahedral geometry or a square pyramidal geometry.
(iii) In ${\text{N}}{{\text{O}}_{\text{2}}}^{\text{ + }}$, nitrogen forms two sigma bonds and two pi-bonds with the oxygen atom. The oxygen atoms are arranged in an angle of ${180^ \circ }$ with the nitrogen atoms. The s-orbital of the nitrogen forms a hybrid with the p-orbital of the nitrogen atom, thereby forming two $sp$ hybridized hybrid orbitals, having a linear geometry.
(iv)${\text{N}}{{\text{H}}_{\text{4}}}^{\text{ + }}$ has the total of $8$ valence electrons. Due to the presence of positive charge, nitrogen has $4$ valence electrons and hydrogen has one in every four hydrogen atoms. The four valence electrons in nitrogen make sigma bonds with the four hydrogen atoms, thereby making a tetrahedral geometry and having $s{p^3}$ hybridized orbitals.
53. Match the species in Column${\text{I}}$with the type of geometry/shape in Column${\text{II}}$.
Column I | Column II | ||
(i) | ${H_3}{O^ + }$ | (a) | Linear |
(ii) |
| (b) | Angular |
(iii) | $ClO_2^ -$ | (c) | Tetrahedral |
(iv) | $NH_4^ + $ | (d) | Trigonal bipyramidal |
(e) | Pyramidal |
Ans:
Column I | Column II | ||
(i) | ${H_3}{O^ + }$ | (e) | Pyramidal |
(ii) | $HC \equiv CH$ | (a) | Linear |
(iii) | $ClO_2^ -$ | (b) | Angular |
(iv) | $NH_4^ + $ | (c) | Tetrahedral |
(i)The shape of ${{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}$ is pyramidal this structure has the total $8$ valence electrons, six in oxygen atom, three in each hydrogen atoms and one electron is lost due to positive charge. Out of five valence electrons in oxygen three form a sigma bond with the hydrogen atom and a lone pair is left on the oxygen atom. Although the four electron pairs make a tetrahedral geometry but to the lone pair of electrons, lone-pair bond-pair repulsion occurs which distorts the shape and makes it pyramidal.
(ii)The ethyne molecule has a linear geometry, because the two carbon atoms make two pi bonds with each other and one sigma bond with the hydrogen atom. The carbon and hydrogen atoms are $sp$ hybridized.
(iii)${\text{Cl}}{{\text{O}}_{\text{2}}}^ -$ has $20$ valence electrons in its structure. Chlorine atoms make one pi-bond and two sigma bonds with the oxygen atoms. The pi-bond is in resonance and hence ${\text{Cl}}{{\text{O}}_{\text{2}}}^ -$ has two resonating structures. Two lone pairs of electrons are left on the oxygen atom and hence, due to lone pair-lone pair repulsion the bond angle reduces and the shape becomes linear.
(iv) ${\text{N}}{{\text{H}}_{\text{4}}}^{\text{ + }}$ has the total of $8$ valence electrons. Due to the presence of positive charge, nitrogen has $4$ valence electrons and hydrogen has one in every four hydrogen atoms. The four valence electrons in nitrogen make sigma bonds with the four hydrogen atoms, thereby making a tetrahedral geometry and having $s{p^3}$ hybridized orbitals.
54.Match the species in Column${\text{I}}$with the bond order in Column${\text{II}}$.
Column${\text{I}}$ | Column${\text{II}}$ | ||
(i) | $NO$ | (a) | $1.5$ |
(ii) | $CO$ | (b) | $2.0$ |
(iii) | ${O_2}^ -$ | (c) | $2.5$ |
(iv) | ${O_2}$ | (d) | $3.0$ |
Ans:
Column${\text{I}}$ | Column${\text{II}}$ | ||
(i) | ${\text{NO}}$ | (c) | $2.5$ |
(ii) | ${\text{CO}}$ | (d) | $3.0$ |
(iii) | ${{\text{O}}_{\text{2}}}^ -$ | (a) | $1.5$ |
(iv) | ${{\text{O}}_{\text{2}}}$ | (b) | $2.0$ |
(i)The electronic configuration of ${\text{NO}}$ is:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\Pi 2{p_x}^2,\Pi 2{p_y}^2,\sigma 2{p_z}^2,{\Pi ^*}2{p_x}^1$
The bond order of ${\text{NO}}$ will be:
$BO = \dfrac{1}{2}[{N_b} - {N_a}]$
$BO = \dfrac{1}{2}[10 - 5] = 2.5$
(ii)The electronic configuration of ${\text{CO}}$ is:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\Pi 2{p_x}^2,\Pi 2{p_y}^2,\sigma 2{p_z}^2$
The bond order of ${\text{CO}}$ will be:
$BO = \dfrac{1}{2}[{N_b} - {N_a}]$
$BO = \dfrac{1}{2}[10 - 4] = 3.0$
(iii)The electronic configuration of ${{\text{O}}_{\text{2}}}^ -$ is:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_x}^2,\Pi 2{p_y}^2,{\Pi ^*}2{p_x}^2,{\Pi ^*}2{p_y}^1$
$BO = \dfrac{1}{2}[{N_b} - {N_a}]$
$BO = \dfrac{1}{2}[10 - 7] = 1.5$
(iv)The electronic configuration of ${{\text{O}}_{\text{2}}}$ is:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_x}^2,\Pi 2{p_y}^2,{\Pi ^*}2{p_x}^1,{\Pi ^*}2{p_y}^1$
$BO = \dfrac{1}{2}[{N_b} - {N_a}]$
$BO = \dfrac{1}{2}[10 - 6] = 2.0$
55.Match the items given in Column${\text{I}}$with examples given in Column${\text{II}}$.
Column${\text{I}}$ | Column${\text{II}}$ | ||
(i) | Hydrogen bond | (a) | $C$ |
(ii) | Resonance | (b) | $LiF$ |
(iii) | Ionic bond | (c) | ${H_2}$ |
(iv) | Covalent solid | (d) | $HF$ |
(e) | ${O_3}$ |
Ans:
Column${\text{I}}$ | Column${\text{II}}$ | ||
(i) | Hydrogen bond | (d) | ${\text{HF}}$ |
(ii) | Resonance | (e) | ${{\text{O}}_3}$ |
(iii) | Ionic bond | (b) | ${\text{LiF}}$ |
(iv) | Covalent solid | (a) | ${\text{C}}$ |
(i)In hydrogen fluoride ${\text{HF}}$, hydrogen forms strong hydrogen bonds with fluorine because of the electronegativity of fluorine. Fluorine is more electronegative than hydrogen and thus the electrons in fluorine are more stabilized and they cannot be easily donated to an acceptor.
(ii)Ozone is a neutral molecule which is stabilized through resonance. The negative charge created by an extra electron is delocalized by resonance through terminal oxygen atoms.
(iii)In lithium fluoride ${\text{LiF}}$, the lithium metal has a positive charge $( + 1)$ and is a cation. The fluoride ion has a negative charge $( - 1)$ and is an anion. These two oppositely charged ions are held together by an ionic bond.
(iv)Carbon is a known covalent solid. The two allotropes of carbon, diamond and graphite are covalent network solids with different geometries. Due to the presence of covalent bonds in graphite and diamond, they have very high melting points and are poor conductors of heat and electricity.
56.Match the shape of molecules in Column${\text{I}}$with the type of hybridization in Column${\text{II}}$.
Column${\text{I}}$ | Column${\text{II}}$ | ||
(i) | Tetrahedral | (a) | $s{p^2}$ |
(ii) | Trigonal | (b) | $sp$ |
(iii) | Linear | (c) | $s{p^3}$ |
Ans:
Column${\text{I}}$ | Column${\text{II}}$ | ||
(i) | Tetrahedral | (c) | $s{p^3}$ |
(ii) | Trigonal | (a) | $s{p^2}$ |
(iii) | Linear | (b) | $sp$ |
(i)A tetrahedral molecule has four electron pairs and these make sigma bonds with each other. The s and p orbitals overlap with each other thus forming a $s{p^3}$ hybridized molecule.
(ii)There are two possibilities for the central atom to have $s{p^2}$ hybridization. Either all the bonds are in place i.e, the bond pairs are all sigma bonds or pi bonds or there are only two bonds and one lone pair of electrons.
(iii)The $sp$ hybridization involves the mixing of the valence electrons in s orbital with another valence electron in the p orbital which yields two $sp$ orbitals which are oriented in a linear geometry.
ASSERTION AND REASON TYPE:
In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
57.Assertion (A): Sodium chloride formed by the action of chlorine gas on sodium metal is a stable compound.
Reason (R): This is because sodium and chloride ions acquire octet in sodium chloride formation.
(i)A and R both are correct, and R is the correct explanation of A.
(ii)A and R both are correct, but R is not the correct explanation of A.
(iii)A is true but R is false.
(iv)A and R both are false.
Ans:Both the statements given in assertion and reason are correct and the reason given is the correct explanation for the assertion. The sodium chloride formed by the action of chlorine gas on the sodium metal has a complete octet. The valence shells of both the atoms are fulfilled, that is why attains an octet.
Correct Answer: Option (i)
58.Assertion (A): Though the central atom of both $N{H_3}$ and${H_2}O$ molecules are $s{p^3}$ hybridised, yet $H - N - H$ bond angle is greater than that of $H - O - H$ .
Reason (R): This is because nitrogen atom has one lone pair and oxygen atom has two lone pairs.
(i)A and R both are correct, and R is the correct explanation of A.
(ii)A and R both are correct, but R is not the correct explanation of A.
(iii)A is true but R is false.
(iv)A and R both are false.
Ans: The statements given in the assertion and reason, both are correct and the reason given is the correct explanation for the assertion.
In ${\text{N}}{{\text{H}}_{\text{3}}}$ only one lone pair of electron is present and due to that lone pair-bond pair repulsion occurs which forms a distorted tetrahedral geometry (pyramidal). In ${{\text{H}}_{\text{2}}}{\text{O}}$ two lone pairs of electrons are present on the oxygen atom and due to this lone pair-lone pair repulsion occurs which shifts the bonds and forms a bent geometry. The lone pair-lone pair repulsion is more than the lone pair-bond pair repulsion. Thus, bond angle in ${\text{H}} - {\text{N}} - {\text{H}}$ is more than that of ${\text{H}} - {\text{O}} - {\text{H}}$.
Correct Answer: Option (i)
59.Assertion (A): Among the two$O - H$ bonds in${H_2}O$ molecule, the energy required to break the first$O - H$bond and the other$O - H$bond is the same.
Reason (R): This is because the electronic environment around oxygen is the same even after breakage of one$O - H$bond.
(i)A and R both are correct, and R is the correct explanation of A.
(ii)A and R both are correct, but R is not the correct explanation of A.
(iii)A is true but R is false.
(iv)A and R both are false.
Ans: Both the statements given in the assertion and reason are false.
The energy required to break the first ${\text{O}} - {\text{H}}$ bond is not same than that of the second ${\text{O}} - {\text{H}}$ bond in water molecule, that means the bond enthalpies of the two ${\text{O}} - {\text{H}}$ are different. This is because the electronic environment around the oxygen atom in water molecules is not the same even after the ${\text{O}} - {\text{H}}$ bond breaks.
Correct Answer: Option (iv)
LONG ANSWER TYPE:
60.Answer the following questions:
(i)Discuss the significance/ applications of dipole moment.
Ans: The significance of dipole moment is as follows:
(a)Dipole moment is used to predict the nature of the molecules. A molecule having zero dipole moment is considered as a nonpolar molecule and a molecule having a specific dipole moment is considered as polar in nature.
(b)Dipole moment is important in determining the shape of a molecule. The shape of a molecule will not be symmetrical if it has a specific dipole moment, it may be bent or angular. A linear molecular which is symmetrical has a zero dipole moment.
(c)Dipole moment is helpful in the comparison of the polarities of the molecules. If the value of the dipole moment is higher, more will be the polarity of the molecule and vice-versa.
(d)Dipole moment also determines if a molecule is in cis- or trans- position, because cis-isomers have higher dipole moment than trans-isomers.
(e)Dipole moment measurements help in differentiating in the ortho-, meta-, and para-isomers. The dipole moment of para-isomers is zero and the dipole moment of ortho-isomers is more than meta-isomers.
(ii)Represent diagrammatically the bond moments and the resultant dipole moment in $C{O_2}$,$N{F_3}$ and $CHC{l_3}$ .
Ans: According to the below shown diagrams, the bond moments and dipole moment of the following molecules is:
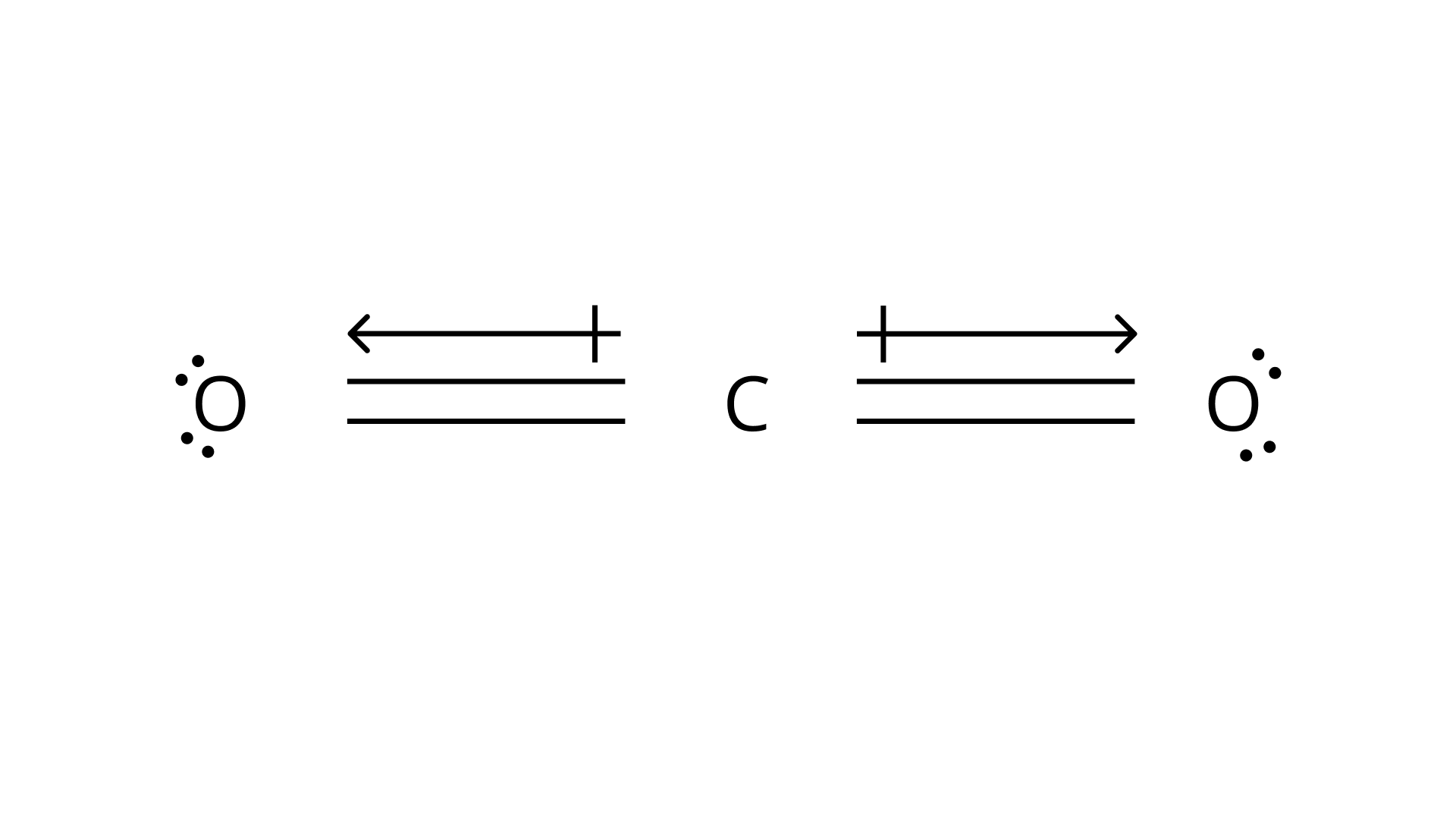
For ${\text{C}}{{\text{O}}_{\text{2}}}$ molecule:
Carbon dioxide has a linear geometry, hence in the above structure the dipole of one ${\text{C}} - {\text{O}}$ is cancelled by another ${\text{C}} - {\text{O}}$. So, the dipole moment of ${\text{C}}{{\text{O}}_{\text{2}}}$ is zero.
For ${\text{N}}{{\text{F}}_{\text{3}}}$molecule:
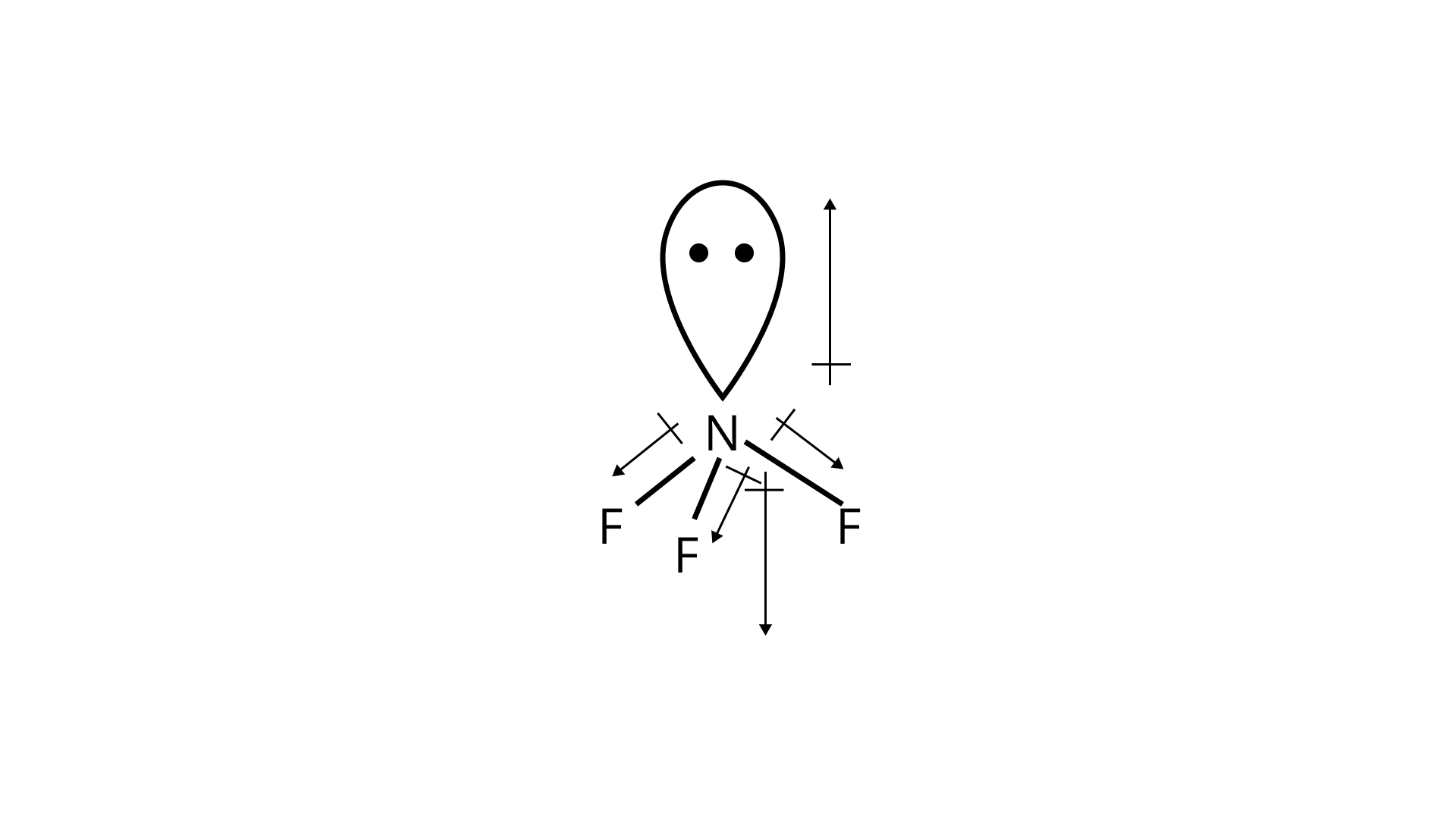
In nitrogen trifluoride, fluorine is more electronegative than hydrogen. The lone pair of electrons on nitrogen atoms also influences the dipole moment of the structure. The dipole moment of ${\text{N}}{{\text{F}}_{\text{3}}}$ was found out to be $0.23D$. In the above molecule, the dipole in ${\text{F}} - {\text{N}}$ weakens the dipole induced by the lone pair of electrons. Thus the vector sum of the four dipole moments is low.
For ${\text{CHC}}{{\text{l}}_{\text{3}}}$molecule:
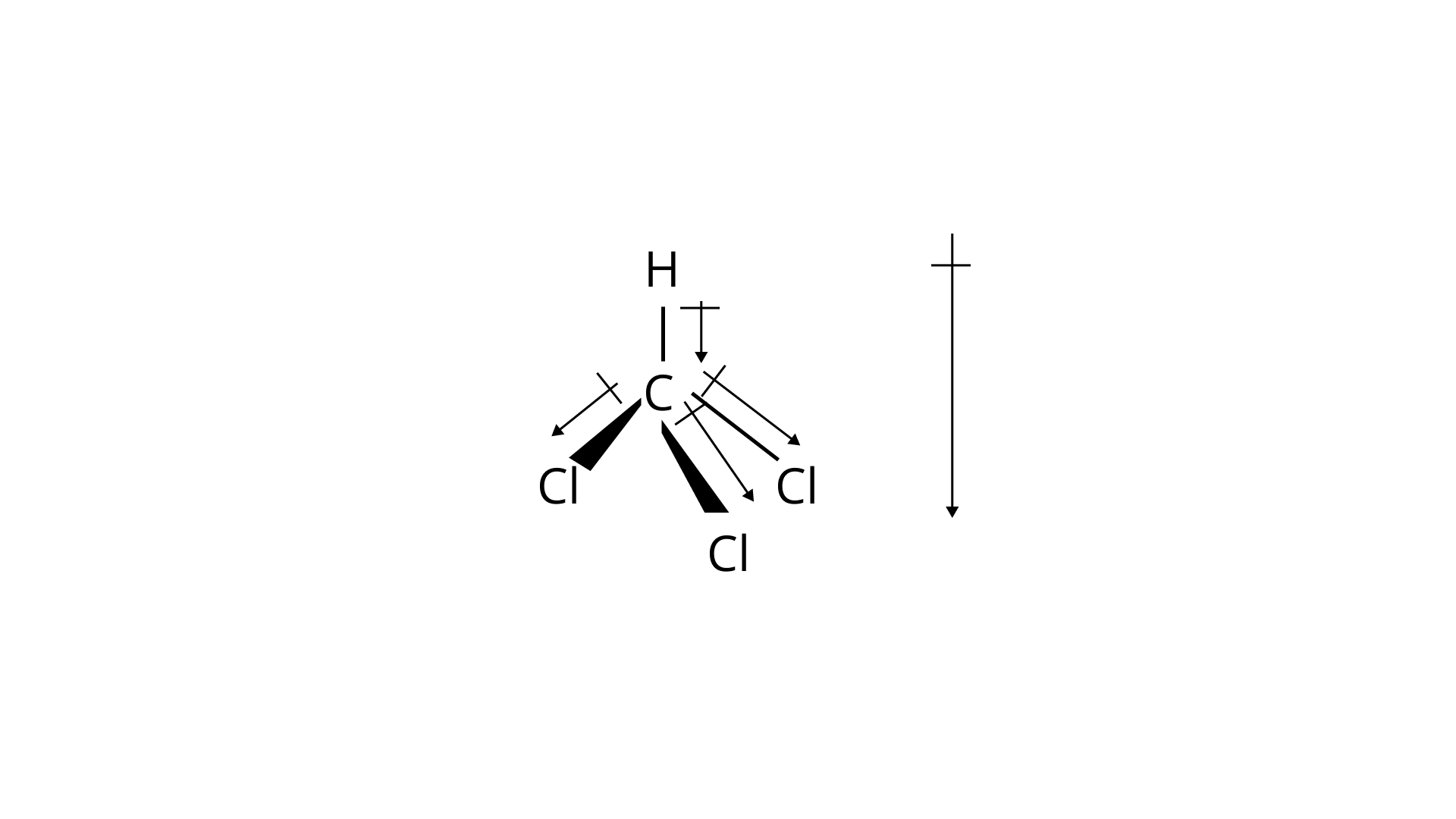
The two terminal chlorine atoms are cancelled with each other, the net dipole moment for chloroform was found out to be $1.04D$.
61. Use the molecular orbital energy level diagram to show that ${N_2}$ would be expected to have a triple bond,${F_2}$, a single bond and $N{e_2}$, no bond.
Ans: Formation of $\mathrm{N}_{2}$ molecule: Electronic Configuration
$\sigma 1 s^{2}<\sigma^{*} 1 s^{2}<\sigma 2 s^{2}<\sigma * 2 s^{2}<\left[\pi 2 p_{x}^{2}=\pi 2 p_{x}^{2}\right]<<2 p_{z}^{2}$
$\text { Bond order }=\left(N_{b}-N_{a}\right) / 2=(10-4) / 2=3$
Bond order indicates the number of bonds in a diatomic molecule. Triple bond $\mathrm{N}=\mathrm{N}$.
Formation of $\mathrm{F}_{2}$ molecule: Electronic Configuration
$\sigma 1 s^{2}<\sigma * 1 s^{2}<\sigma 2 s^{2}<\sigma * 2 s_{z}^{2}, \sigma * 2 p z^{2}<\left[\pi 2 p_{x}^{2}=\pi 2 p_{x}^{2}\right]<\left[\pi^{*} 2 p_{x}^{2}\right.$
$\left.=\pi^{*} 2 p_{x}^{2}\right]<\sigma * 2 p z$
Bond order $=\left(N_{b}-N_{a}\right) / 2=(10-8) / 2=1 .$ Single bond F-F.
Formation of $\mathrm{Ne}_{2}$ molecule: Electronic Configuration
$\sigma 1 s^{2}<\sigma * 1 s^{2}<\sigma 2 s^{2}<\sigma * 2 s^{2}<\sigma * 2 p z^{2}<\left[\pi 2 p_{x}^{2}=\pi 2 p_{x}^{2}\right]<\left[\pi * 2 p_{x}^{2}\right.$
$\left.=\pi^{*} 2 p_{x}^{2}\right]<\sigma * 2 p_{z}^{2}$
$\text { Bond order }=\left(N_{b}-N_{a}\right) / 2=(10-10) / 2=0$
Hence there will be no bond formation in between the neon atoms.
62. Briefly describe the valence bond theory of covalent bond formation by taking an example of hydrogen. How can you interpret energy changes taking place in the formation of dihydrogen?
Ans:Introduced by Hietler and London in $1927$, valence bond theory was further developed by Pauling. Valence bond theory (VBT) describes the information about the atomic orbitals, it tells about the electronic configuration of elements, the overlap criteria of atomic orbitals and also the hybridization of atomic orbitals is depicted by the valence bond theory.
A and B are two hydrogen atoms which are approaching each other and these hydrogen atoms have the nuclei ${N_A},{N_B}$ respectively, the electrons in the two hydrogen atoms are represented as ${e_A},{e_B}$ respectively. When the distance between these two hydrogen atoms is large, then there will be no interaction between them. New attractive and repulsive forces begin to generate as these two hydrogen atoms approach each other.
Attractive forces arise between these two hydrogen atoms. As a result of these attractive forces, the two atoms come closer to each other. It has been found experimentally that the magnitude of new attractive forces was more than the new repulsive forces. Due to this when the two atoms approach each other the potential energy decreases.
Therefore, a stage occurs, where the net force of attraction balances the net repulsive forces and a state of minimum energy is acquired by the system. At this stage the two hydrogen atoms form a bond together, thus forming a stable bond.
When the two hydrogen atoms form a bond, the energy is released, the hydrogen molecule formed is more stable than the individual hydrogen atom. The energy released during the formation of the hydrogen molecule is called bond enthalpy.
63. Describe hybridisation in the case of $PC{l_5}$ and$S{F_6}$. The axial bonds are longer as compared to equatorial bonds in $PC{l_5}$ whereas in $S{F_6}$ both axial bonds and equatorial bonds have the same bond length. Explain.
Ans: Hybridisation in the case of ${\text{PC}}{{\text{l}}_{\text{5}}}$:
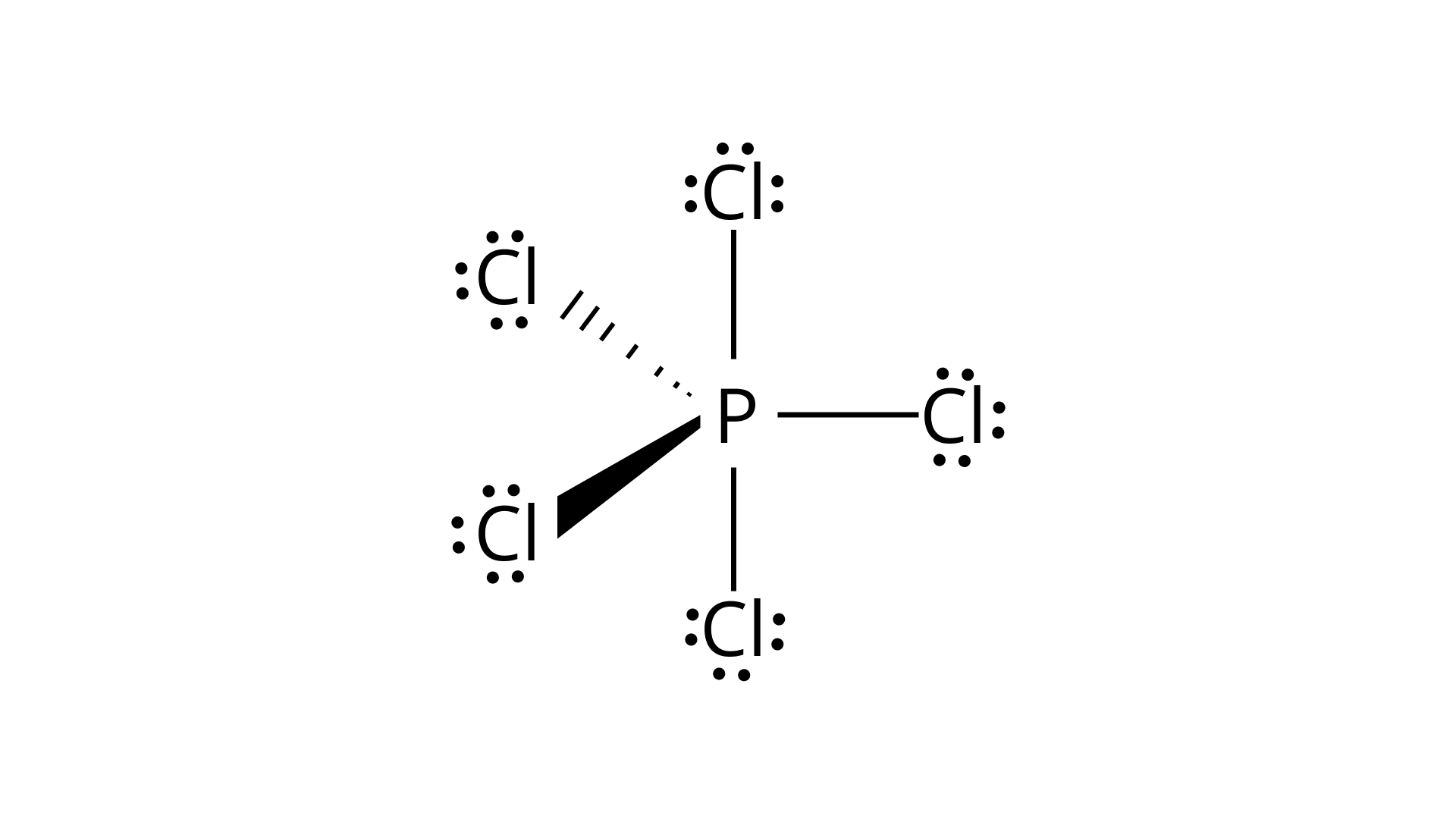
The hybridisation of phosphorus ${\text{PC}}{{\text{l}}_{\text{5}}}$ is $s{p^3}d$ and it has a trigonal bipyramidal geometry. The axial bonds in ${\text{PC}}{{\text{l}}_{\text{5}}}$ are slightly longer as compared to the equatorial bonds because axial bonds experience greater repulsive forces from other bonds as compared to the equatorial bonds.
Hybridisation in the case of ${\text{S}}{{\text{F}}_{\text{6}}}$:
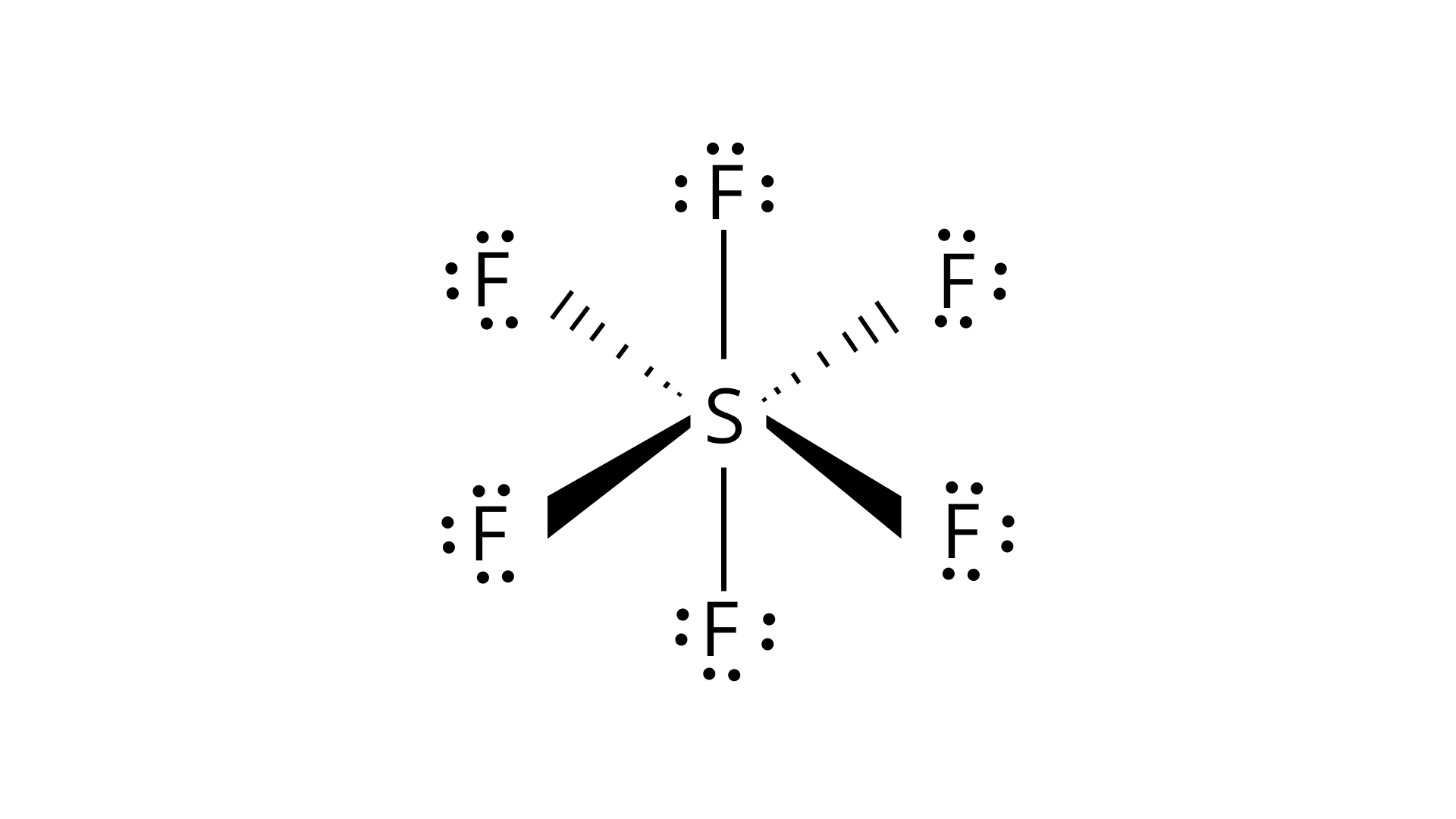
The hybridisation of sulphur in ${\text{S}}{{\text{F}}_{\text{6}}}$ is $s{p^3}{d^2}$ and the molecule has an octahedral geometry. The bond length of axial as well as the equatorial bonds are similar because all the bonds in octahedral geometry experience similar repulsive forces from other bonds.
64. Answer the following:
(i) Discuss the concept of hybridisation. What are its different types in a carbon atom.
Ans: Different types of hybridization in a carbon atom are:
(a)$sp$ hybridization: The carbon atoms forming triple bonds with each other determine$sp$ hybridized carbon. (${\text{C}} \equiv {\text{C}}$ ).
(b)$s{p^2}$ hybridization: The carbon atoms forming double bonds with each other determine$s{p^2}$ hybridized carbon. (${\text{C}} = {\text{C}}$)
(c)$s{p^3}$ hybridization: The carbon atoms forming single bonds with each other determine $s{p^3}$ hybridized carbon. (${\text{C}} - {\text{C}}$)
(ii) What is the type of hybridisation of carbon atoms marked with star.
(a)
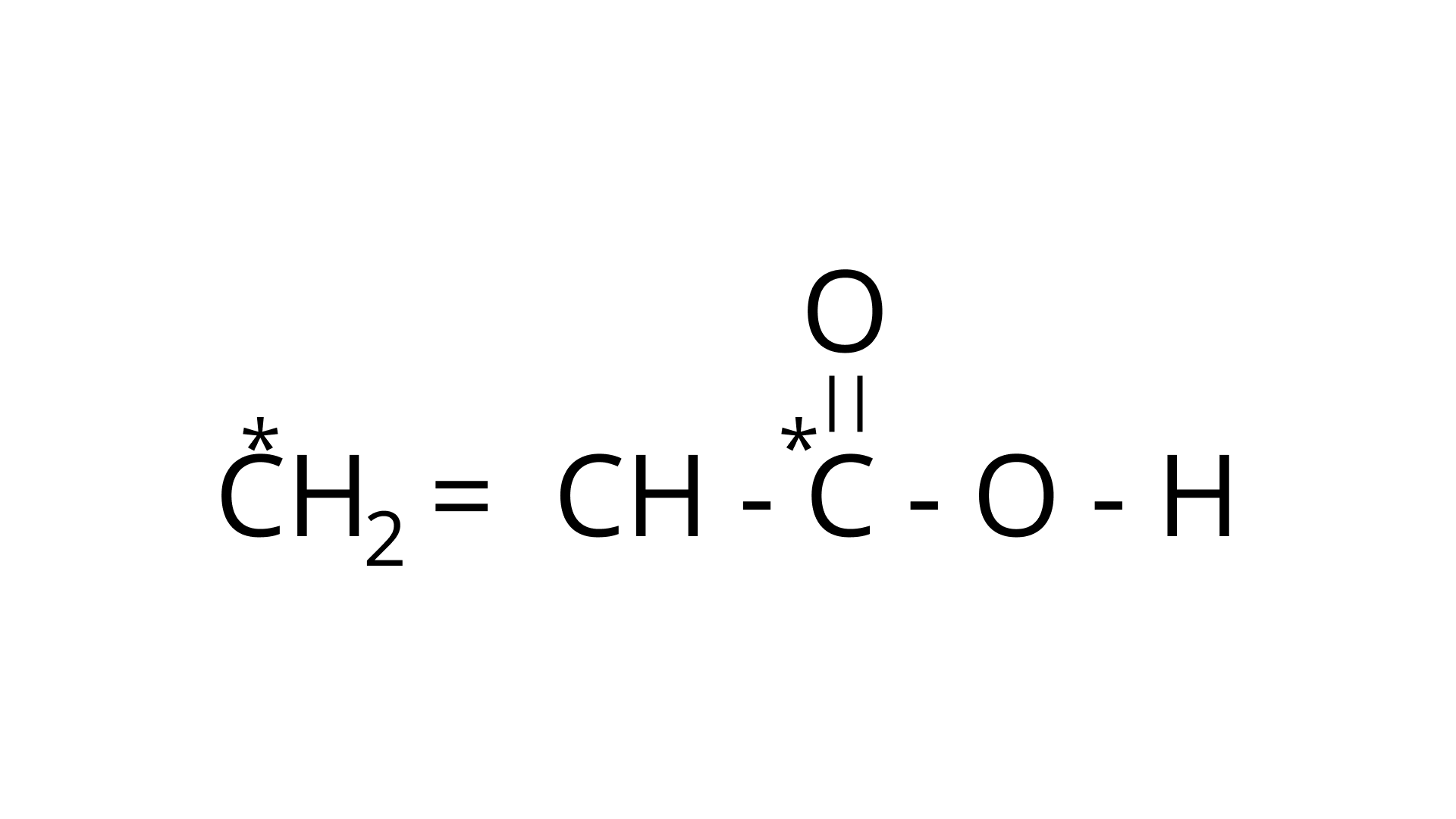
Ans: The type of hybridization marked with the star in the given structure is:
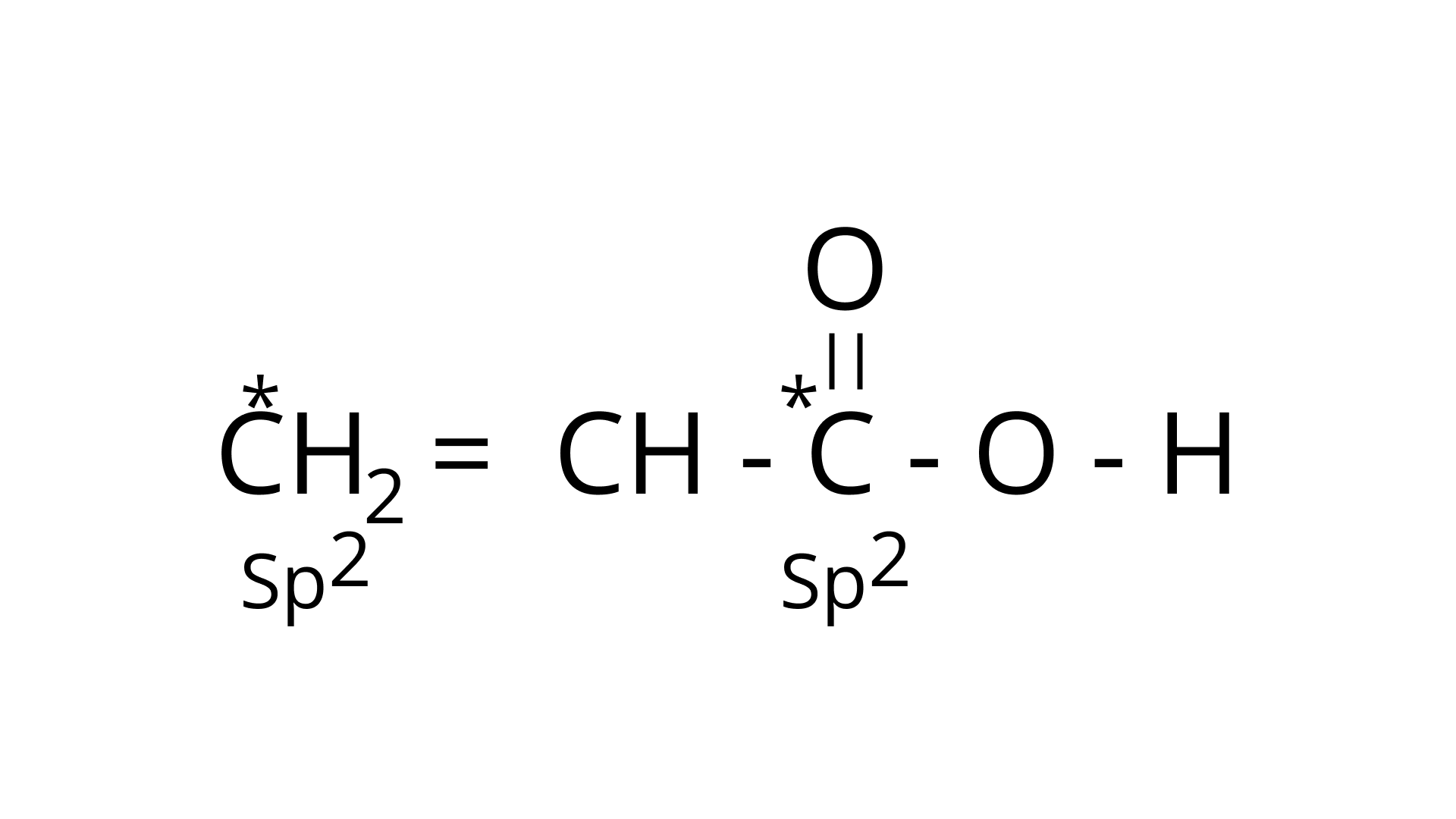
(b) $\mathrm{CH}_{3}-\stackrel{*}{\mathrm{CH}}_{2}-\mathrm{OH}$
Ans: The type of hybridization marked with the star in the given structure is:
$\mathrm{CH}_{3}-\stackrel{*}{\mathrm{CH}}_{2}-\mathrm{OH}$
The starred $\mathrm{C}$ atom is $s p^{3}$ hybridised.
(c)
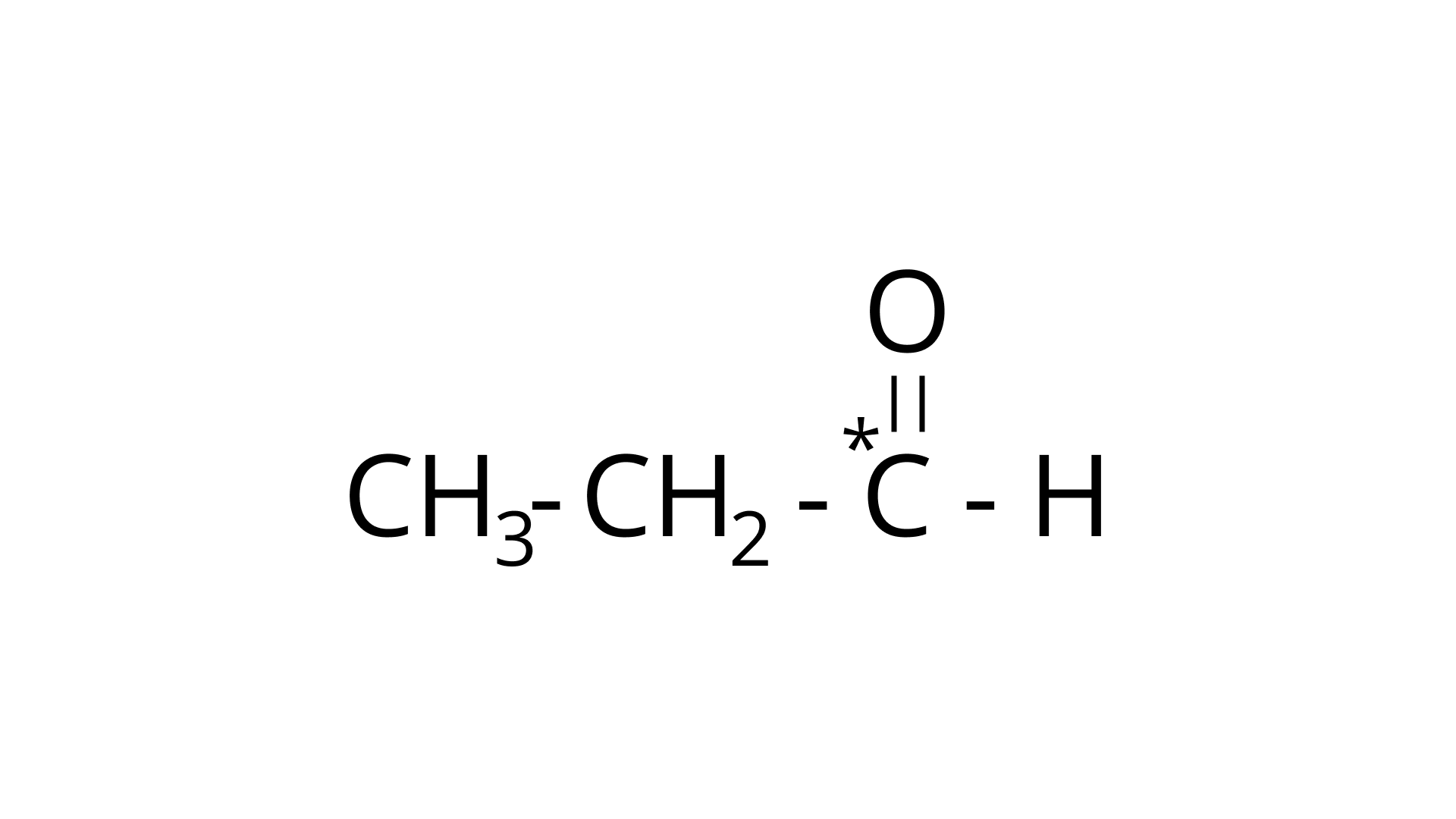
Ans: The type of hybridization marked with the star in the given structure is:
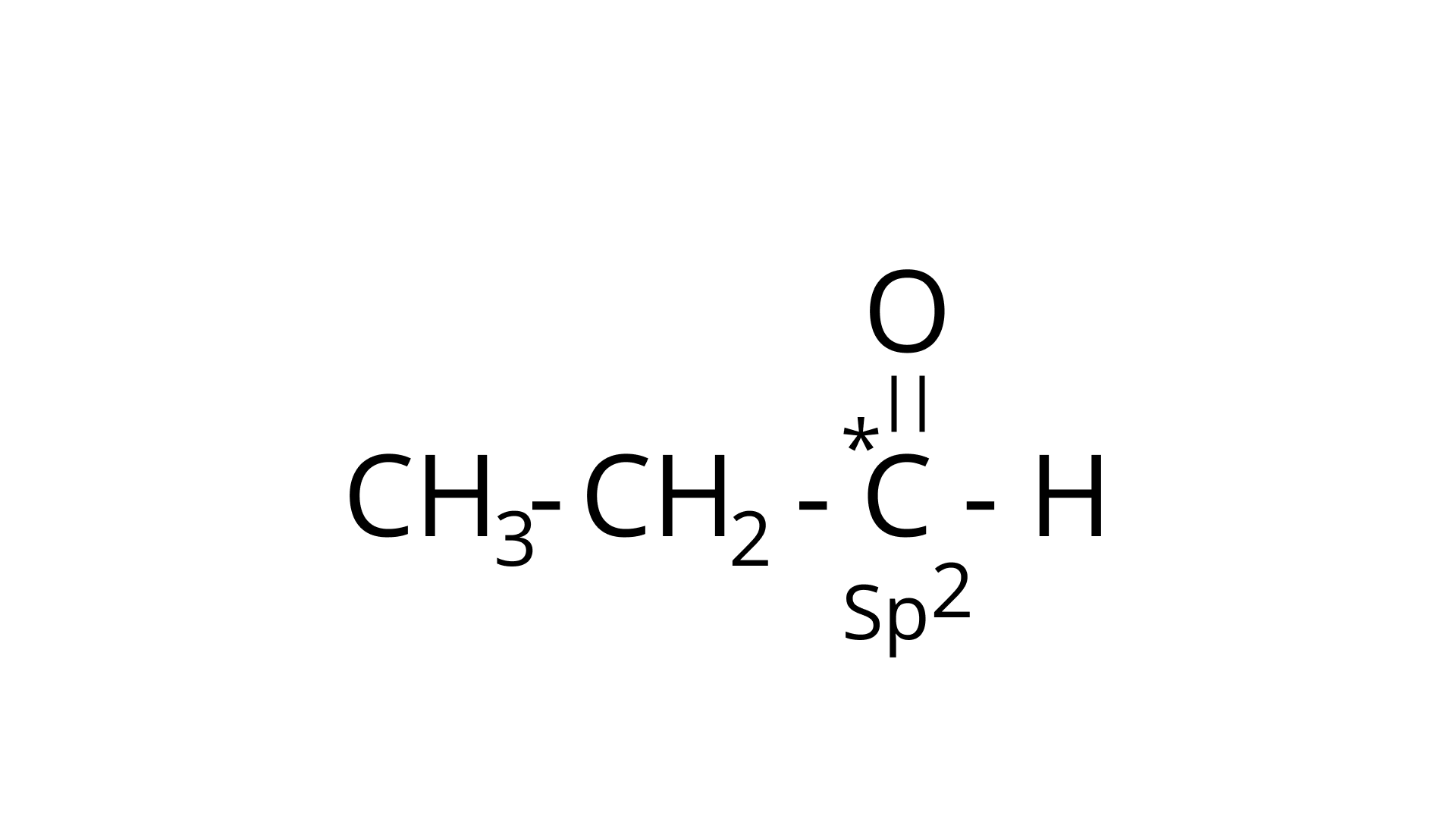
(d) $\stackrel{*}{\mathrm{CH}_{3}}-\mathrm{CH}=\mathrm{CH}-\mathrm{CH}_{3}$
Ans: The type of hybridization marked with the star in the given structure is:
$\mathrm{CH}_{3}-\mathrm{CH}=\mathrm{C} \mathrm{H}-\mathrm{CH}_{3}$
The starred $\mathrm{C}$ atom is $s p^{3}$ hybridised.
(e) $\mathrm{CH}_{3}-\stackrel{*}{\mathrm{C}} \equiv \mathrm{CH}$
Ans: The type of hybridization marked with the star in the given structure is:
$\mathrm{CH}_{3}-\stackrel{\circ}{\mathrm{C}} \equiv \mathrm{CH}$
The starred C atom is $s p^{2}$ hybridised.
Comprehension given below is followed by some multiple choice questions. Each question has one correct option. Choose the correct option.
Molecular orbitals are formed by the overlap of atomic orbitals. Two atomic orbitals combine to form two molecular orbitals called bonding molecular orbital (BMO) and anti bonding molecular orbital (ABMO). Energy of anti bonding orbital is raised above the parent atomic orbitals that have combined and the energy of the bonding orbital is lowered than the parent atomic orbitals. Energies of various molecular orbitals for elements hydrogen to nitrogen increase in the order : $\sigma 1s < {\sigma ^*}1s < \sigma 2s < {\sigma ^*}2s < (\Pi 2{p_x} \approx \Pi 2{p_y}) < \sigma 2{p_z} < ({\Pi ^*}2{p_x} \approx {\Pi ^*}2{p_y}) < {\sigma ^*}2{p_z}$ and for oxygen and fluorine order of energy of molecular orbitals is given below: $\sigma 1s < {\sigma ^*}1s < \sigma 2s < {\sigma ^*}2s < \sigma 2{p_z} < (\Pi 2{p_x} \approx \Pi 2{p_y}) < ({\Pi ^*}2{p_x} \approx {\Pi ^*}2{p_y}) < {\sigma ^*}2{p_z}$Different atomic orbitals of one atom combine with those atomic orbitals of the second atom which have comparable energies and proper orientation. Further, if the overlapping is head on, the molecular orbital is called ‘Sigma’, ($\sigma $) and if the overlap is lateral, the molecular orbital is called ‘pi’, ($\Pi $). The molecular orbitals are filled with electrons according to the same rules as followed for filling of atomic orbitals. However, the order for filling is not the same for all molecules or their ions. Bond order is one of the most important parameters to compare the strength of bonds.
65.Which of the following statements is correct?
(i)In the formation of dioxygen from oxygen atoms $10$ molecular orbitals will be formed.
(ii)All the molecular orbitals in the dioxygen will be completely filled.
(iii)Total number of bonding molecular orbitals will not be same as total number of anti bonding orbitals in dioxygen.
(iv)Number of filled bonding orbitals will be same as number of filled anti bonding orbitals.
Ans: (i)The electronic configuration of dioxygen is:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_x}^2,\Pi 2{p_y}^2,{\Pi ^*}2{p_x}^1,{\Pi ^*}2{p_y}^1$
Only nine molecular orbitals are formed. So, the statement given is incorrect.
(ii)As it can be seen from the electronic configuration of oxygen atom the${\Pi ^*}2{p_x}^1,{\Pi ^*}2{p_y}^1$ are partially. So, the statement given is incorrect.
(iii)The statement given is correct because there are five bonding molecular orbitals and four antibonding molecular orbital in oxygen molecules. Hence, the bonding and antibonding molecular orbitals are no equal.
(iv)The filled bonding orbitals are not the same as the number of filled antibonding orbitals. The filled bonding orbitals are five and only two filled antibonding orbitals are present in dioxygen molecules.
Correct Answer: Option (iii)
66.Which of the following molecular orbitals has maximum number of nodal planes?
(i) ${\sigma ^*}1s$
(ii) ${\sigma ^*}2{p_x}$
(iii) $\Pi 2{p_x}$
(iv) ${\Pi ^*}2{p_y}$
Ans: Nodal plane is a plane which passes through the nucleus and the probability of finding an electron on a nodal plane is zero.
Amongst the above given molecular orbital, only${\Pi ^*}2{p_x}$contains two nodal planes. Rest of the molecular orbitals, ${\sigma ^*}1s$,$\Pi 2{p_x}$,${\Pi ^*}2{p_y}$contains one nodal plane.
Correct Answer: Option (ii)
67.Which of the following pair is expected to have the same bond order?
(i) ${O_2},{N_2}$
(ii) ${O_2}^ + ,{N_2}^ -$
(iii) ${O_2}^ - ,{N_2}^ + $
(iv) ${O_2}^ - ,{N_2}^ -$
Ans:(i)The electronic configuration of ${{\text{O}}_{\text{2}}}$ is:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_x}^2,\Pi 2{p_y}^2,{\Pi ^*}2{p_x}^1,{\Pi ^*}2{p_y}^1$
The bond order of ${{\text{O}}_{\text{2}}}$ will be:
BO = $\dfrac{1}{2}[{N_b} - {N_a}]$
BO = $\dfrac{1}{2}[10 - 6] = 2.0$
The electronic configuration of ${{\text{N}}_{\text{2}}}$:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\Pi 2p{x^2},\Pi 2p{y^2},\sigma 2p{z^2}$
The bond order of ${{\text{N}}_{\text{2}}}$ will be:
${\text{BO}} = \dfrac{1}{2}[{N_b} - {N_a}]$
${\text{BO}} = \dfrac{1}{2}[10 - 4] = 3.0$
So, the bond order of ${{\text{O}}_{\text{2}}},{{\text{N}}_{\text{2}}}$ will not be equal.
(ii)The electronic configuration of ${{\text{O}}_{\text{2}}}^{\text{ + }}$ is:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_x}^2,\Pi 2{p_y}^2,{\Pi ^*}2{p_x}^1$
The bond order of ${{\text{O}}_{\text{2}}}^{\text{ + }}$ will be:
${\text{BO}} = \dfrac{1}{2}[{N_b} - {N_a}]$
${\text{BO}} = \dfrac{1}{2}[10 - 5] = 2.5$
The electronic configuration of ${{\text{N}}_{\text{2}}}^ -$ is:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\Pi 2p{x^2},\Pi 2p{y^2},\sigma 2p{z^2},{\Pi ^*}2{p_x}^1$
The bond order of ${{\text{N}}_{\text{2}}}^ -$ will be:
${\text{BO}} = \dfrac{1}{2}[{N_b} - {N_a}]$
${\text{BO}} = \dfrac{1}{2}[10 - 5] = 2.5$
Hence, the bond order of ${{\text{O}}_{\text{2}}}^ + ,{{\text{N}}_{\text{2}}}^ -$ will be equal.
(iii)The electronic configuration of ${{\text{O}}_{\text{2}}}^ -$ is:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_x}^2,\Pi 2{p_y}^2,{\Pi ^*}2{p_x}^2,{\Pi ^*}2{p_y}^1$
The bond order of ${{\text{O}}_{\text{2}}}^ -$ will be:
${\text{BO}} = \dfrac{1}{2}[{N_b} - {N_a}]$
${\text{BO}} = \dfrac{1}{2}[10 - 7] = 1.5$
The electronic configuration of ${{\text{N}}_{\text{2}}}^{\text{ + }}$ is:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\Pi 2p{x^2},\Pi 2p{y^2},\sigma 2p{z^1}$
The bond order of ${{\text{N}}_{\text{2}}}^{\text{ + }}$ will be:
${\text{BO}} = \dfrac{1}{2}[{N_b} - {N_a}]$
${\text{BO}} = \dfrac{1}{2}[9 - 4] = 2.5$
Hence, the bond order of ${{\text{O}}_{\text{2}}}^ - ,{{\text{N}}_{\text{2}}}^ + $ will not be equal.
(iv)The electronic configuration of ${{\text{O}}_{\text{2}}}^ -$ is:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\sigma 2{p_z}^2,\Pi 2{p_x}^2,\Pi 2{p_y}^2,{\Pi ^*}2{p_x}^2,{\Pi ^*}2{p_y}^1$
The bond order of ${{\text{O}}_{\text{2}}}^ -$ will be:
${\text{BO}} = \dfrac{1}{2}[{N_b} - {N_a}]$
${\text{BO}} = \dfrac{1}{2}[10 - 7] = 1.5$
The electronic configuration of ${{\text{N}}_2}^ -$ is:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\Pi 2p{x^2},\Pi 2p{y^2},\sigma 2p{z^2},{\Pi ^*}2{p_x}^1$
The bond order of ${{\text{N}}_{\text{2}}}^ -$ will be:
${\text{BO}} = \dfrac{1}{2}[{N_b} - {N_a}]$
${\text{BO}} = \dfrac{1}{2}[10 - 5] = 2.5$
Hence, the bond order of ${{\text{O}}_{\text{2}}}^ - ,{{\text{N}}_{\text{2}}}^ -$ will not be equal.
Correct Answer: Option (ii)
68.In which of the following molecules,$\sigma 2{p_z}$molecular orbital is filled after$\Pi 2{p_x}$ and$\Pi 2{p_y}$ molecular orbitals?
(i) ${O_2}$
(ii) $ N{e_2}$
(iii) ${N_2}$
(iv) ${F_2}$
Ans: Molecules from hydrogen to nitrogen shows this type of electronic configuration:
$\sigma 1s < {\sigma ^*}1s < \sigma 2s < {\sigma ^*}2s < (\Pi 2{p_x} \approx \Pi 2{p_y}) < \sigma 2{p_z} < ({\Pi ^*}2{p_x} \approx {\Pi ^*}2{p_y}) < {\sigma ^*}2{p_z}$, in which $\sigma 2{p_z}$ molecular orbital is filled after$\Pi 2{p_x}$ and $\Pi 2{p_x}$.
So, amongst the given elements only nitrogen will show this type of electronic configuration in which $\sigma 2{p_z}$ molecular orbital is filled after $\Pi 2{p_x}$ and $\Pi 2{p_x}$.
The electronic configuration of ${{\text{N}}_{\text{2}}}$ is:
$\sigma 1{s^2},{\sigma ^*}1{s^2},\sigma 2{s^2},{\sigma ^*}2{s^2},\Pi 2p{x^2},\Pi 2p{y^2},\sigma 2p{z^2}$
Correct Answer: Option (iii)
Summary of Chemical Bonding and Molecular Structure
Following are the three major exceptions to the rule of octaves:
Incomplete Octet of the Central Atom - In some compounds, the number of Electrons present around the central Atom is less than eight. It occurs mainly in compounds of those elements in which the number of valence Electrons is less than four.
Odd Electron molecule - In those molecules in which the total number of Electrons is odd, such as nitric oxide (NO) and nitrogen dioxide (NO), not all Atoms obey the octet rule.
Expanded octet: In addition to 35 and 3p orbitals, 3d-orbitals are also available for Bonding in the elements of the third and beyond the period of the periodic table. Many compounds of these elements have more than eight Electrons around the central Atom. This is called an expanded octet. The octet rule does not apply to these compounds. Some Examples of such compounds are PFs, SF 6, H2SO4 and many covalent compounds.
Favourable Factors For Ionic Bond Formation Following Factors Are Favourable For Forming An Ionic Bond
Ionisation Enthalpy:
In the formation of a positive ion or cation, one of the Atoms has to give up Electrons, which requires Ionisation Enthalpy. We know that Ionisation Enthalpy is the amount of energy required to remove the outermost Electron from an isolated gaseous Atom; Therefore, the lower the ionisation enthalpy required, the easier the formation of the cation will be. 5- The alkali metals and alkaline earth metals present in the block generally form cations, because their ionisation enthalpy is relatively low.
Electron Gain Enthalpy:
liberated in the formation of cations. The Electrons are taken up by the other Atoms participating in the formation of the ionic bond. The tendency of Atoms to accept Electrons depends on the Electron gain enthalpy. The amount of energy released by an isolated gaseous Atom to acquire an Electron and become an anion is called Electron gain enthalpy. It is thus clear that the formation of an anion will be easier if the Electron gain enthalpy is more negative. The halogens present in group 17 have the highest tendency to form anion because their Electron gain enthalpy is very negative. Members of the oxygen family (group 16) also tend to form anions, but this is not easily possible; Because energy is required to form divalent anions (02-).
Lattice Energy or Enthalpy:
Ionic compounds occur in the form of crystalline solids and crystals of ionic compounds, cations and anions are arranged regularly in three-dimensional form. Since ions are charged species; Therefore, the energy released in the attraction of ions is called lattice energy or enthalpy. It can be defined as: The energy released when one mole of a crystalline solid is obtained by combining oppositely charged ions is called lattice energy or enthalpy." It is expressed by 'U'.
It is thus clear that the stability of the Ionic Bond or Ionic Compound will be greater if the magnitude of the lattice energy is fixed. In conclusion, if the magnitude of the lattice energy and the negative Electron gain enthalpy is greater than the required ionisation enthalpy, then a stable Chemical bond will be obtained. If they are less then the bond will not be formed.
Electronegativity - The Electronegativity of an element can be defined in such a way that the measure of the tendency of its Atom to attract the shared Electron pair in a covalent bond is called the Electronegativity of the element.
Features of Vedantu's Notes and Solutions
Easy language and eye-catching formatting.
The topics are as per the latest syllabus pattern so that students can revise Chemistry Chapter 4 Chemical Bonding and Molecular Structure Notes in the shortest possible time with maximum accuracy.
NCERT Book for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure Notes are as per CBSE syllabus guidelines.
After studying the NCERT Book for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure Notes, students will not be nervous about heavy books.
These notes will surely save you time during stressful Exam days.
FAQs on NCERT Exemplar for Class 11 Chemistry - Chemical Bonding and Molecular Structure - Free PDF
1. Write the factors favourable for the formation of Ionic Bonds.
Favourable Factors for Ionic Bond Formation Following factors are favourable for forming an ionic bond
(1) Ionization Enthalpy - In the formation of a positive ion or cation, one of the Atoms has to give up Electrons, which requires ionisation enthalpy. We know that ionisation enthalpy is the amount of energy required to remove the outermost Electron from an isolated gaseous Atom; Therefore, the lower the ionisation enthalpy required, the easier the formation of the cation will be. 5- The alkali metals and alkaline earth metals present in the block generally form cations, because their ionisation enthalpy is relatively low.
(2) Electron Gain Enthalpy - liberated in the formation of cations. The Electrons are taken up by the other Atoms participating in the formation of the ionic bond. The tendency of Atoms to accept Electrons depends on the Electron gain enthalpy. The amount of energy released by an isolated gaseous Atom to acquire an Electron and become an anion is called Electron gain enthalpy. It is thus clear that the formation of an anion will be easier if the Electron gain enthalpy is more negative. The halogens present in group 17 have the highest tendency to form anion because their Electron gain enthalpy is very negative. Members of the oxygen family (group 16) also tend to form anions, but this is not easily possible; Because energy is required to form a divalent anion.
(3) Lattice energy or enthalpy- Ionic compounds occur in the form of crystalline solids and crystals of ionic compounds, the cations and anions are arranged regularly in three-dimensional form. Since ions are charged species; Therefore, the energy released in the attraction of ions is called lattice energy or enthalpy. It can be defined as: The energy released when one mole of a crystalline solid is obtained by combining oppositely charged ions is called lattice energy or enthalpy." It is expressed by 'U'.
It is thus clear that the stability of the ionic bond or ionic compound will be greater if the magnitude of the lattice energy is fixed. In conclusion, if the magnitude of the lattice energy and the negative Electron gain enthalpy is greater than the required ionisation enthalpy, then a stable Chemical bond will be obtained. If they are less then the bond will not be formed.
2. How to articulate bond strength in terms of bond order?
If the bond dissociation enthalpy is high then the bond will be stronger and the bond enthalpy increases as the bond order increases. This is clear from the fact that the bond strength and the bond order are proportional to each other. For Example, the bond order of N2 is 3 and its bond enthalpy is 945kJ mol-1. Similarly, the bond order of 02 is 2 and its bond enthalpy is 498 jmol-1. Among these, the N2 bond will be stronger.
3. Define bond-length.
The equilibrium distance between the nuclei of the bonded atoms in a molecule is called the bond length. The bond length values are usually expressed in picometers (1 pm = 10-12 m).
In the ionic compounds, the bond length between two bonded Atoms is obtained by adding their ionic radii. Similarly, in the covalent compounds, the bond length between two bonded Atoms is obtained by adding up their covalent (Atomic) radii. As the bond order increases, the bond strength will also increase.
4. State the important applications of dipole moment.
Important Applications of Dipole Moment Following are some important applications of dipole moment
(1) Predicting the nature of the molecules - Molecules with a fixed dipole moment are polar, whereas molecules with zero dipole moment are nonpolar. Thus, BeF2 (u = 0 D) is nonpolar, while H2O (u = 1.84D) is polar.
(2) Predicting the Molecular Structure of the molecules - we know that Atomic gases; For Example, the dipole moment of inert gases etc. is zero, that is, they are non-polar, but diAtomic molecules are polar and nonpolar; Like - H2O2. Adi is nonpolar (u = 0) and CO is polar. The Structure of these molecules is also linear.
TriAtomic molecules are also polar and non-polar. CO2, CS2, etc. are nonpolar; Because for them = 0; Therefore, the Structure of these molecules is linear.
Water molecule is polar because = 184D; Hence its Structure cannot be linear. It has an angular Structure and each O-H bond has an angle of 104°5'. Similarly, H25 and SO2 also have angular Structures; Because the values for these are 0.90 D and 1.71 D respectively. Molecules having four Atomicities are also polar and nonpolar. 4 = 0 for the BCl3 molecule i.e. it is nonpolar. Therefore, its Structure is similar to that of an isosceles triangle.
(3) Determining the polarity of the bonds - The ionic property of polarity in a covalently bonded compound depends on the Electronegativity of the Atoms of the elements used in the formation of that bond. Thus, the difference in Electronegativity and dipole moment of the Atoms of the bond.
The difference in the Electronegativity of the Atoms of the bond.
(4) Determining the ionic percentage of the bonds - Dipole moment values help in finding the ionic percentage of polar bonds. It is the ratio of the observed dipole moment or the experimentally determined dipole moment to the total Electron transfer dipole moment (theoretical).
5. What do you understand about polar covalent bonds?
Polar covalent compound - In many molecules, one Atom is more negatively Electronegative than the other Atom, so it tends to attract the Electron pair of the covalent bond towards itself so that the Electron pair is correctly located at the centre of the molecule. does not remain in the Atom, but is attracted to the Atom of the more negatively Electronegative element. Because of this one. A positive charge (which has low Electronegativity) on one Atom and a negative charge (which has a high negative Electronegativity) on another Atom is produced. The molecule thus obtained is called a polar covalent compound and the bond formed in it is called a polar covalent bond.

























