NCERT Exemplar for Class 12 Chemistry - The d and f Block Elements - Free PDF Download
Free PDF download of NCERT Exemplar for Class 12 Chemistry Chapter 8 - The d and f Block Elements solved by expert Chemistry teachers on Vedantu.com as per NCERT (CBSE) Book guidelines. All Chapter 8 - The d and f Block Elements Exercise questions with solutions to help you to revise complete syllabus and score more marks in your Examinations.
Inorganic Chemistry is definitely one of the most important subjects for students in Class 12. Surprisingly, it is also one of the easiest subjects to score full marks in. The chances of students losing their marks in Inorganic Chemistry are much less than them losing marks in any other subject. Inorganic Chemistry is based on a lot of theory and hence it does not threaten a lot of students for the most part. This, however, does not mean that Inorganic Chemistry requires any less attention or time.
This free PDF from Vedantu is a detailed solution set for the Class 12 NCERT Exemplar Book Exercises of the Chapter The d and f Block Elements that can help students to get a good idea on how to write answers in the final tests and also help them in their endeavor of scoring well in competitive examinations like JEE, NEET, etc.
Access NCERT Exemplar Solutions for Class 12 Chemistry Chapter 8 - The d- and f- Block Elements
Exercise
MULTIPLE CHOICE QUESTION (TYPE-I):
1. Electronic configuration of a transition element ${\text{X}}$ in +3 oxidation state is \[\left[ {Ar} \right] 3{d^5}\]. What is its atomic number?
(A) 25
(B) 26
(D) 27
(D) 24
Ans: (B) 26
Positive oxidation states indicate the loss of electrons from the atom. If X is in +3 oxidation state, then three electrons have been removed from it. Find the atomic number of the parent atom, we will add three in the given electronic number. i.e.
${{\text{X}}^{{\text{3 + }}}}{\text{(Z = 23) = }}\left[ {{\text{Ar}}} \right]{\text{ 3}}{{\text{d}}^{\text{5}}}$
${\text{X = 23 + 3 = 26}}$
2. The electronic configuration of \[Cu\left( {II} \right)\] is \[3{d^9}\] whereas that of \[Cu\left( I \right)\] is \[3{d^{10}}\]. Which of the following is correct?
(a) \[Cu\left( {II} \right)\] is more stable .
(b) \[Cu\left( {II} \right)\] is less stable
(c) \[Cu\left( I \right)\] and \[Cu\left( {II} \right)\] are equally stable
(d) Stability of \[Cu\left( {II} \right)\] and \[Cu\left( I \right)\] depends on the nature of copper salts.
Ans: (A) \[Cu\left( {II} \right)\] is more stable .
Electronic configuration of \[{\text{Cu}}\]is \[\left[ {{\text{Ar}}} \right]{\text{ 3}}{{\text{d}}^{{\text{10}}}}{\text{ 4}}{{\text{s}}^{\text{1}}}\]
\[{\text{Cu}}\left( {\text{I}} \right)\] - \[\left[ {{\text{Ar}}} \right]{\text{ 3}}{{\text{d}}^{{\text{10}}}}{\text{ 4}}{{\text{s}}^0}\]
\[{\text{Cu}}\left( {{\text{II}}} \right)\]- \[\left[ {{\text{Ar}}} \right]{\text{ 3}}{{\text{d}}^9}{\text{ 4}}{{\text{s}}^0}\]
Despite the fact that\[{\text{Cu}}\left( {\text{I}} \right)\] has fully filled 3d-orbital but \[{\text{Cu}}\left( {{\text{II}}} \right)\] is more stable than \[{\text{Cu}}\left( {\text{I}} \right)\] due to the greater effective nuclear charge of \[{\text{Cu}}\left( {{\text{II}}} \right)\] as nucleus has to hold 17 electrons rather than 18 electrons like in \[{\text{Cu}}\left( {\text{I}} \right)\].
3. Metallic radii of some transition elements are given below. Which of these elements will have the highest density?
Element | Fe | Co | Ni | Cu |
Metallic radii/pm | 126 | 125 | 125 | 128 |
(A) Fe
(B) Ni
(C) Co
(D) Cu
Ans: (D) Cu
Density is mass by volume. As we move from left to right for a long period, the atomic radii decrease. Hence, volume decreases. Also, an increase in atomic masses is observed.
So overall the density increases out of the above option from iron to copper, copper will have the highest density.
4. Generally, transition elements form coloured salts due to the presence of unpaired electrons. Which of the following compounds will be coloured in solid-state?
(A) $A{g_2}S{O_4} $
(B) $Cu{F_2} $
(C) $Zn{F_2} $
(D) $C{u_2}C{l_2}$
Ans: (B) $Cu{F_2} $
$\left( {\text{A}} \right){\text{ A}}{{\text{g}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{ (A}}{{\text{g}}^{\text{ + }}}) \to {\text{ 5}}{{\text{d}}^{{\text{10}}}}{\text{ 6}}{{\text{s}}^{\text{0}}}$
$\left( {\text{B}} \right){\text{ Cu}}{{\text{F}}_{\text{2}}}{\text{ (C}}{{\text{u}}^{{\text{2 + }}}}) \to {\text{ 3}}{{\text{d}}^9}{\text{ 4}}{{\text{s}}^{\text{0}}}$
$\left( {\text{C}} \right){\text{ Zn}}{{\text{F}}_{\text{2}}}{\text{ (Z}}{{\text{n}}^{{\text{2 + }}}}{\text{)}} \to {\text{ 3}}{{\text{d}}^{{\text{10}}}}{\text{ 4}}{{\text{s}}^{\text{0}}}$
$\left( {\text{D}} \right){\text{ C}}{{\text{u}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}{\text{ (C}}{{\text{u}}^{\text{ + }}}) \to {\text{ 3}}{{\text{d}}^{{\text{10}}}}{\text{ 6}}{{\text{s}}^{\text{0}}}$
Unpaired electrons present in any compound impart colour to the salt of transition metal. Only \[{\text{Cu}}{{\text{F}}_{\text{2}}}\] has unpaired electrons in its \[{\text{3d}}\]orbital that’s why it is white coloured in its solid-state while rest of the salts are colourless.
5. In addition to a small amount of \[KMn{O_4}\] to concentrate \[{H_2}S{O_4}\], a green oily compound is obtained which is highly explosive in nature. Identify the compound from the following:
(A) $M{n_2}{O_7} $
(B) $Mn{O_2}$
(C) $MnS{O_4} $
(D) $M{n_2}{O_3}$
Ans: (A) $M{n_2}{O_7} $
${\text{2KMn}}{{\text{O}}_{\text{4}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(conc}}{\text{.) }} \to {\text{ M}}{{\text{n}}_{\text{2}}}{{\text{O}}_{\text{7}}}{\text{ + 2KHS}}{{\text{O}}_{\text{4}}}{\text{ + }}{{\text{H}}_{\text{2}}}{\text{O}}$
Manganese heptoxide is an acid anhydride of permanganic acid \[\left( {{\text{ HMn}}{{\text{O}}_{\text{4}}}} \right)\]. It is a dangerous oxidiser, volatile and highly reactive liquid.
6. The magnetic nature of elements depends on the presence of unpaired electrons. Identify the configuration of the transition element, which shows the highest magnetic moment.
(A) $3{d^7} $
(B) $3{d^5} $
(C) $3{d^8} $
(D) $3{d^2}$
Ans: (B) $3{d^5} $
Magnetic moment of an electron/dipole moment is caused by its intrinsic properties of spin and electric charge. It depends upon the number of unpaired electrons in its valence shell. The more the number of unpaired electrons, the greater will be the value of magnetic moment.
${\text{3}}{{\text{d}}^{\text{7}}}{\text{ = 3 unpaired electrons }}$
${\text{3}}{{\text{d}}^{\text{5}}}{\text{ = 5 unpaired electrons}}$
${\text{3}}{{\text{d}}^{\text{8}}}{\text{ = 2 unpaired electrons }}$
${\text{3}}{{\text{d}}^{\text{2}}}{\text{ = 2 unpaired electrons}}$
Out of all, \[{\text{3}}{{\text{d}}^{\text{5}}}\] has five unpaired electrons that is maximum and hence it has the highest magnetic moment.
7. Which of the following oxidation states is common for all lanthanides?
(A) $ + 2 $
(B) $ + 3$
(C) $ + 4 $
(D) $ + 5$
Ans: (B) +3
Out of all +3 is the most common oxidation state for all the lanthanide elements.
8. Which of the following reactions are disproportionation reactions?
i. $C{u^ + } \xrightarrow{{}}C{u^{2 + }} + Cu $
ii. $3Mn{O^{4-}} + 4{H^ + } \xrightarrow{{}} 2Mn{O_4}^-- + Mn{O_2} + 2{H_2}O $
iii. $2KMn{O_4} \xrightarrow{{}} {K_2}Mn{O_4} + Mn{O_2} + {O_2} $
iv. $2Mn{O_4}^- + 3M{n^{2 + }} + 2{H_2}O \xrightarrow{{}} 5Mn{O_2} + 4{H^ + }$
(A)i,ii
(B)i,ii,iii
(C)ii,iii,iv
(D) i,iv
Ans: (A)i,ii
Disproportionation/redox reaction is a reaction in which one compound is oxidised as well as reduced at the same time.
$\left( {\text{A}} \right){\text{ C}}{{\text{u}}^{\text{ + }}}{\text{ }} \to {\text{ C}}{{\text{u}}^{{\text{2 + }}}}{\text{ + Cu }}$
${\text{ ( + 1) ( + 2) (0) }}$
$\left( {\text{B}} \right){\text{ 3Mn}}{{\text{O}}^{{\text{4--}}}}{\text{ + 4}}{{\text{H}}^{\text{ + }}}{\text{ }} \to {\text{ 2Mn}}{{\text{O}}_{\text{4}}}^{\text{--}}{\text{ + Mn}}{{\text{O}}_{\text{2}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O }}$
${\text{ ( + 6) ( + 7) ( + 4)}}$
9. When \[KMn{O_4}\] solution is added to oxalic acid solution, the decolourisation is slow in the beginning but becomes instantaneous after some time because
(A) \[{\text{}}C{O_2}\] is formed as the product
(B) Reaction is exothermic
(C) \[Mn{O_4}\] catalyses the reaction
(D) \[M{n^{2 + }}\] acts as auto catalyst
Ans: (D)\[M{n^{2 + }}\] acts as auto catalyst
\[{\text{M}}{{\text{n}}^{{\text{2 + }}}}\] formed in the reaction acts as an autocatalyst. Auto catalyst is a compound that is one of the products that are formed in the reaction itself catalyses the reaction.
Reaction: \[{\text{2Mn}}{{\text{O}}^{{\text{4 - }}}}{\text{ + 16}}{{\text{H}}^{\text{ + }}}{\text{ + 5}}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}^{{\text{2 - }}} \to {\text{ 2M}}{{\text{n}}^{{\text{2 + }}}}{\text{ + 10C}}{{\text{O}}_{\text{2}}}{\text{ + 8H2O}}\]
10. There are 14 elements in the actinide series. Which of the following elements does-not belong to this series?
(A) $ U $
(B) $ Np $
(C) $Tm $
(D) $ Fm$
Ans: (C) $Tm $
In the periodic table, actinides lie from atomic number 90 to 103.
Thulium is not an actinide; it is a lanthanide.
11. \[KMn{0_4}\] acts as an oxidizing agent in acidic medium. The number of moles of \[KMn{0_4}\] that will be needed to react with one mole of sulphide ions in acidic solution is
(A) \[\frac{2}{5}\]
(B) \[\frac{3}{5}\]
(C) \[\frac{4}{5}\]
(D) \[\frac{1}{5}\]
Ans: (A) \[\frac{2}{5}\]
\[{\text{2Mn}}{{\text{O}}_{\text{4}}}^{\text{ - }}{\text{ + 5}}{{\text{S}}^{{\text{2 - }}}}{\text{ + 16}}{{\text{H}}^{\text{ + }}}{\text{ }} \to {\text{ 2M}}{{\text{n}}^{{\text{2 + }}}}{\text{ + 5S + }}{{\text{H}}_{\text{2}}}{\text{O}}\]
From the reaction,
For 5 moles of \[{\text{S}}\] , two moles of \[{\text{KMn}}{{\text{O}}_{\text{4}}}\] were required.
Therefore, for one mole of \[{\text{S}}\] , the number of moles of \[{\text{KMn}}{{\text{O}}_{\text{4}}}\] required will be = \[\frac{2}{5}\].
12. Which of the following is amphoteric oxide?
\[M{n_2}{O_7}, CrO3, C{r_2}{O_3}, CrO, {V_2}O{5_,} {V_2}{O_4}\]
(A) ${V_2}{O_5}, C{r_2}{O_3} $
(B) $ M{n_2}{O_7}, Cr{O_3} $
(C) $CrO, {V_2}{O_5} $
(D) ${V_2}{O_5}, {V_2}{O_4}$
Ans: (A) ${V_2}{O_5}, C{r_2}{O_3} $
Oxides in the lower oxidation state are basic and in the higher oxidation state they are acidic. They are amphoteric in the intermediate oxidation state. Therefore, \[{{\text{V}}_{\text{2}}}{{\text{O}}_{\text{5}}}{\text{, C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{ }}\]are amphoteric in nature.
13. Gadolinium belongs to the 4f series. Its atomic number is 64. Which of the following is the correct electronic configuration of gadolinium?
(A) $\left[ {Xe} \right] 4{f^7} 5{d^1} 6{s^2} $
(B) $\left[ {Xe} \right] 4{f^6} 5{d^2} 6{s^2} $
(C) $\left[ {Xe} \right] 4{f^8} 6{d^2} $
(D) $ \left[ {Xe} \right] 4{f^9} 5{s^1} $
Ans: (A) $\left[ {Xe} \right] 4{f^7} 5{d^1} 6{s^2} $
Gadolinium belongs to the 4f series; it has atomic no.= 64. It has extra stability due to a half-filled 4f subshell.
\[{\text{Gd }}\left( {Z = 64} \right){\text{ }} \to {\text{ }}\left[ {{\text{Xe}}} \right]{\text{ 4}}{{\text{f}}^{\text{6}}}{\text{ 5}}{{\text{d}}^{\text{2}}}{\text{ 6}}{{\text{s}}^{\text{2}}}{\text{ }}\]
14. Interstitial compounds are formed when small atoms are trapped inside the crystal lattice of metals. Which of the following is not the characteristic property of interstitial compounds?
(A) They have high melting points in comparison to pure metals
(B) They are very hard
(C) They retain metallic conductivity
(D) They are chemically very reactive.
Ans: (D) They are chemically very reactive.
Interstitial compounds/alloys are substances that are formed when a small atom like carbon, hydrogen, boron, nitrogen can occupy space in their lattices. All the properties mentioned above are true for interstitial compounds except (D), they are chemically inert.
15. The magnetic moment is associated with its spin angular momentum and orbital angular momentum. Spin only magnetic moment value of \[C{r^{3 + }}\]ion is
(A) 2.87 B.M.
(B) 3.87 B.M.
(C) 3.47 B.M.
(D) 3.57 B.M.
Ans: (B) 3.87 B.M.
${\text{Cr }} \to {\text{ (Z = 24)}}$
${\text{C}}{{\text{r}}^{{\text{3 + }}}}{\text{ }} \to {\text{ (Z = 21)}}$
${\text{1}}{{\text{s}}^{\text{2}}}{\text{ 2}}{{\text{s}}^{\text{2}}}{\text{ 2}}{{\text{p}}^{\text{6}}}{\text{ 3}}{{\text{s}}^{\text{2}}}{\text{ 3}}{{\text{p}}^{\text{6}}}{\text{ 3}}{{\text{d}}^{\text{3}}}{\text{ 4}}{{\text{s}}^{\text{0}}}$
${\text{n = 3 ( 3 unpaired electrons )}}$
${{\mu = }}\sqrt {{\text{n ( n + 2 )}}} $
${{\mu = }}\sqrt {{\text{3 ( 3 + 2 )}}} $
${{\mu = }}\sqrt {{\text{3( 5 )}}} $
${{\mu = }}\sqrt {15{\text{ }}} {\text{ = 3}}{\text{.87 B}}{\text{.M}}{\text{.}}$
16.\[{\text{}}KMn{O_4}\] acts as an oxidizing agent in alkaline medium. When alkaline \[{\text{}}KMn{O_4}\] is treated with \[{\text{KI}}\], iodide ion is oxidized to
(A) ${I_2} $
(B) $I{O^ - } $
(C) $I{O_3}^ - $
(D) $I{O_4}^ - $
Ans: (C) \[{\text{I}}{{\text{O}}_{\text{3}}}^{\text{ - }}\]
Iodide is oxidised to iodate.
${\text{2KMn}}{{\text{O}}_{\text{4}}}{\text{ + KI + }}{{\text{H}}_{\text{2}}}{\text{O }} \to {\text{ 2KOH + 2Mn}}{{\text{O}}_{\text{2}}}{\text{ + KI}}{{\text{O}}_{\text{3}}}$
${\text{ iodide iodate}}$
17. Which of the following statements is not correct?
(A) Copper liberates hydrogen from acids
(B) In its higher oxidation states, manganese forms stable compounds with oxygen and fluorine
(C) \[M{n^{3 + }}\]and \[C{o^{3 + }}\] are oxidizing agents in aqueous solution
(D) \[T{i^{2 + }}\]and \[C{r^{2 + }}\]a re reducing agents in aqueous solution
Ans: (A) is incorrect.
Copper lies below hydrogen in electrochemical series and hence cannot liberate hydrogen from acids.
${\text{Cu + 2}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{ }} \to {\text{ CuS}}{{\text{O}}_{\text{4}}}{\text{ + S}}{{\text{O}}_{\text{2}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}}$
18. When acidified \[{K_2}C{r_2}{O_7}\] solution is added to \[S{n^{2 + }}\] salts, \[S{n^{2 + }}\] changes to
(A) $Sn $
(B) $S{n^{3 + }} $
(C) $S{n^{4 + }} $
(D) $S{n^ + }$
Ans: (C) $S{n^{4 + }} $
\[{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}^{{\text{2 - }}}{\text{ + 14}}{{\text{H}}^{\text{ + }}}{\text{ + 3S}}{{\text{n}}^{{\text{2 + }}}}{\text{ }} \to {\text{ 2C}}{{\text{r}}^{{\text{3 + }}}}{\text{ + 3S}}{{\text{n}}^{{\text{4 + }}}}{\text{ + 7}}{{\text{H}}_{\text{2}}}{\text{O}}\]
In the above redox reaction, oxidation of \[{\text{S}}{{\text{n}}^{{\text{2 + }}}}\] is taking place. It gets converted to \[{\text{S}}{{\text{n}}^{{\text{4 + }}}}\] whereas, reduction of chromium is occurring from +6 oxidation state to +3.
19. Highest oxidation state of manganese in fluoride is +4 \[\left( {Mn{F_4}} \right)\]but highest oxidation state in oxides is +7 \[\left( {M{n_2}{O_7}} \right)\] because
(A) fluorine is more electronegative than oxygen
(B) fluorine does not possess d-orbitals
(C) fluorine stabilizes lower oxidation state
(D) in covalent compounds fluorine can form single bond only while oxygen forms double bond
Ans: (D) in covalent compounds fluorine can form single bond only while oxygen forms double bond
Oxygen has the capacity to form multiple bonds which enables it to form a variety of covalent compounds.
In \[\left( {{\text{M}}{{\text{n}}_{\text{2}}}{{\text{O}}_{\text{7}}}} \right)\] also, 6 oxygen are doubly bonded to two manganese atoms and one oxygen is forming bridge between two.
While in \[\left( {{\text{Mn}}{{\text{F}}_{\text{4}}}} \right)\], four fluorine atoms are singly bonded to manganese atom giving it a +4 oxidation state.
Therefore, due to capability of oxygen to have multiple bonds in covalent compounds, manganese is having higher oxidation state of +7 in \[\left( {{\text{M}}{{\text{n}}_{\text{2}}}{{\text{O}}_{\text{7}}}} \right)\] .
20. Although zirconium belongs to 4d transition series and hafnium to 5d
transition series even then they show similar physical and chemical properties because
(A) both belong to d-block
(B) both have same number of electrons
(C) both have similar atomic radius
(D) both belong to the same group of the periodic table
Ans: (C) both have similar atomic radius
As an effect of lanthanide contraction, zirconium and hafnium have similar radii of 160pm and 159 pm respectively. Due to this similarity in their size, they show similar physical and chemical properties.
Lanthanide contraction: Because the elements in Row 3 have 4f electrons. These electrons do not shield well, causing a greater nuclear charge. This greater nuclear charge has a greater pull on the electrons and results in the decrease in their size and atomic radii.
21. Why is \[HCl\] not used to make the medium acidic in oxidation reactions of \[KMn{O_4}\]in acidic medium?
(A) Both\[HCl\] and \[KMn{O_4}\] act as oxidizing agents.
(B) \[KMn{O_4}\] oxidises \[HCl\] into \[C{l_2}\]which is also an oxidizing agent.
(C) \[KMn{O_4}\] is a weaker oxidizing agent than \[HCl\].
(D) \[KMn{O_4}\] acts as a reducing agent in the presence of \[HC{\text{l}}\].
Ans: (B) \[KMn{O_4}\] oxidises \[HCl\] into \[C{l_2}\]which is also an oxidizing agent.
\[{\text{HCl}}\] is not used in titrations involving\[{\text{KMn}}{{\text{O}}_{\text{4}}}\] as it produces unsatisfactory results. When\[{\text{HCl}}\] is added to \[{\text{KMn}}{{\text{O}}_{\text{4}}}\], it itself acts as reducing agent and coverts \[{\text{HCl}}\] to \[{\text{C}}{{\text{l}}_{\text{2}}}\].
MULTIPLE CHOICE QUESTIONS (TYPE-II)
22. Generally, transition elements and their salts are coloured due to the presence of unpaired electrons in metal ions. Which of the following compounds are coloured?
(A) $KMn{O_4} $
(B) $Ce{\left( {S{O_4}} \right)_2} $
(C) $TiC{l_4} $
(D) $C{u_2}C{l_2}$
Ans: (A) & (B)
Due to charge transfer phenomenon, which can be understood, for an instant, an electron is transferred from oxygen to the central metal atom, where oxidation state of metal is reduced by one and oxygen gets converted from \[{{\text{O}}^{\text{ - }}}\]to \[{{\text{O}}^{{\text{2 - }}}}\].
This transfer imparts Colour to the compound.
23. Transition elements show magnetic moments due to spin and orbital motion of electrons. Which of the following metallic ions have almost the same spin only magnetic moment?
(A) $C{o^{2 + }} $
(B) $C{r^{2 + }} $
(C) $M{n^{2 + }} $
(D) $C{r^{3 + }}$
Ans: (A) & (D)
The magnetic moment of an electron/dipole moment is caused by its intrinsic properties of spin and electric charge. It depends upon the number of unpaired electrons in its valence shell. The more the number of unpaired electrons, the greater will be the value of the magnetic moment.
\[{\text{C}}{{\text{o}}^{{\text{2 + }}}}{\text{ }}\]and \[{\text{C}}{{\text{r}}^{{\text{3 + }}}}\]have same unpaired electrons i.e. 3 unpaired electrons. Hence they have the same spin only magnetic moment.
24. In the form of dichromate, Cr(VI) is a strong oxidizing agent in acidic medium
but \[Mo\] (VI) in \[Mo{O_3}\] and \[W\] (VI) in \[W{O_3}\] are not because
(A) \[Cr\] (VI) is more stable than \[Mo\] (VI) and \[W\] (VI)
(B) \[Mo\] (VI) and \[{\text{W}}\] (VI) are more stable than \[Cr\] (VI)
(C) higher oxidation states of heavier members of group-6 of transition series are more stable
(D) lower oxidation states of heavier members of group-6 of transition series are more stable.
Ans: (B) and (C)
For the heavier metals of d-block, higher oxidation state is more favourably stable than the lower oxidation state. For example: \[{\text{Mo}}\] (VI) and \[{\text{W}}\] (VI) are more stable than \[{\text{Cr}}\] (VI). For lighter d-block elements like chromium, +3 oxidation state is more stable. So, \[{\text{Cr}}\] (VI) in dichromate act as a strong oxidising agent by reducing itself from +6 to +3 oxidation states While \[{\text{Mo}}\] (VI) and \[{\text{W}}\] (VI) does not.
25. Which of the following actinoids show oxidation states up to +7?
(A) $Am $
(B) $Pu $
(C) $U $
(D) $Np$
Ans: (B) & (D)
Actinides show a variety of oxidation states like +3, +5, +6 and +7.
From the given option, plutonium and neptunium show all oxidation states up to +7.
26. General electronic configuration of actinides is \[\left( {n -- 2} \right){f^{1 - 14}}\left( {n -- 1 } \right){d^{0 - 2}}n{s^2}\]. Which of the following actinoids have one electron in 6d orbital?
(A) U (Atomic no. 92)
(B) Np (Atomic no. 93)
(C) Pu (Atomic no. 94)
(D) Am (Atomic no. 95)
Ans: (A) and (B)
Electronic configuration:
${}_{{\text{92}}}{\text{U - 5}}{{\text{f}}^{\text{3}}}{\text{ 6}}{{\text{d}}^{\text{1}}}{\text{ 7}}{{\text{s}}^{\text{2}}}$
${}_{{\text{93}}}{\text{Np - 5}}{{\text{f}}^4}{\text{ 6}}{{\text{d}}^{\text{1}}}{\text{ 7}}{{\text{s}}^{\text{2}}}$
27. Which of the following lanthanoids show +2 oxidation state besides the characteristic oxidation state +3 of lanthanides?
(A) $Ce $
(B) $Eu $
(C) $Yb $
(D) $Ho$
Ans: (B) and (C)
${\text{Ce (Z = 57) = }}\left[ {{\text{Xe}}} \right]{\text{ 4}}{{\text{f}}^{\text{5}}}{\text{ 5}}{{\text{d}}^{\text{0}}}{\text{ 6}}{{\text{s}}^{\text{2}}}{\text{ , O}}{\text{.S}}{\text{. = + 3, + 4}}$
${\text{Eu (Z = 63) = }}\left[ {{\text{Xe}}} \right]{\text{ 4}}{{\text{f}}^{\text{7}}}{\text{ 5}}{{\text{d}}^{\text{0}}}{\text{ 6}}{{\text{s}}^{\text{2}}}{\text{, O}}{\text{.S}}{\text{. = + 2, + 3}}$
${\text{Yb (Z = 70) = }}\left[ {{\text{Xe}}} \right]{\text{ 4}}{{\text{f}}^{{\text{14}}}}{\text{ 5}}{{\text{d}}^{\text{0}}}{\text{ 6}}{{\text{s}}^{\text{2}}}{\text{, O}}{\text{.S}}{\text{. = + 2, + 3}}$
${\text{Ho (Z = 67) = }}\left[ {{\text{Xe}}} \right]{\text{ 4}}{{\text{f}}^{{\text{11}}}}{\text{ 5}}{{\text{d}}^{\text{0}}}{\text{ 6}}{{\text{s}}^{\text{2}}}{\text{, O}}{\text{.S}}{\text{. = + 3}}$
28. Which of the following ions show higher spin only magnetic moment value?
(A) $T{i^{3 + }} $
(B) $M{n^{2 + }} $
(C) $F{e^{2 + }} $
(D) $C{o^{3 + }}$
Ans: (B) and (C)
Magnetic moment of an electron/dipole moment is caused by its intrinsic properties of spin and electric charge. It depends upon the number of unpaired electrons in its valence shell. The more the number of unpaired electrons, the greater will be the value of magnetic moment.
Electronic configuration:
${\text{T}}{{\text{i}}^{{\text{3 + }}}}{\text{ = 3}}{{\text{d}}^{\text{1}}}{\text{ = 1 unpaired electron}}$
${\text{M}}{{\text{n}}^{{\text{2 + }}}}{\text{ = 3}}{{\text{d}}^{\text{5}}}{\text{ = 5 unpaired electrons}}$
${\text{F}}{{\text{e}}^{{\text{2 + }}}}{\text{ = 3}}{{\text{d}}^{\text{6}}}{\text{ = 4 unpaired electrons}}$
${\text{C}}{{\text{o}}^{{\text{2 + }}}}{\text{ = 3}}{{\text{d}}^{\text{7}}}{\text{ = 3 unpaired electrons}}$
Out of all given options, \[{\text{M}}{{\text{n}}^{{\text{2 + }}}}\]and \[{\text{F}}{{\text{e}}^{{\text{2 + }}}}\] have unpaired electrons in number of five and four respectively. Hence, have higher spin only magnetic moment.
29. Transition elements form binary compounds with halogens. Which of the following elements will form \[M{F_3}\] type compounds?
(A) $Cr $
(B) $Co $
(C) $Cu $
(D) $Ni$
Ans: (A) and (B)
\[{\text{Cr}}\] and \[{\text{Co}}\] form tri halide compounds.
30. Which of the following will not act as oxidizing agents?
(A) $Cr{O_3}$
(B) $Mo{O_3}$
(C) $ W{O_3} $
(D) $Cr{O_4}^{2 - }$
Ans: (B) and (C)
A compound act as oxidising agent when it’s central atom is reduced to its lower oxidation state. This occurs only when the lower oxidation state of metal is more stable than the higher oxidation states.
In metal \[{\text{W}}{{\text{O}}_{\text{3}}}\] and \[{\text{Cr}}{{\text{O}}_{\text{4}}}^{{\text{2 - }}}\], both \[{\text{W}}\] and \[{\text{Cr}}\] are stable in their higher oxidation state and won’t get reduced to their lower oxidation state. Therefore, will not act as oxidising agents.
31. Although +3 is the characteristic oxidation state for lanthanide but cerium
also shows +4 oxidation state because
(A) it has variable ionization enthalpy
(B) it has a tendency to attain noble gas configuration
(C) it has a tendency to attain \[{{\text{f}}^{{^\circ }}}\] configuration
(D) it resembles \[P{b^{4 + }}\]
Ans: (B) and (C)
Electronic configuration of cerium:
${\text{Ce - 4}}{{\text{f}}^{\text{1}}}{\text{5}}{{\text{d}}^{\text{1}}}{\text{6}}{{\text{s}}^{\text{2}}}$
${\text{C}}{{\text{e}}^{4 + }} - {\text{ 4}}{{\text{f}}^{\text{0}}}{\text{5}}{{\text{d}}^0}{\text{6}}{{\text{s}}^0}$
Lead has the tendency to lose its four outer electrons to attain Noble gas configuration and this removal of electrons corresponds to the \[{{\text{f}}^{{^\circ }}}\] configuration and cause it to be stable in +4 oxidation state.
SHORT ANSWER TYPE:
32. Why does copper not replace hydrogen from acids?
Ans: Copper lies below hydrogen in electrochemical series which means it has positive standard reduction potential i.e. \[{\text{ + 0}}{\text{.34 V}}\]. and hence cannot liberate hydrogen from acids.
33. Why \[{E^\circ }\] value for \[Mn\], \[Ni\] and \[Zn\] are more negative than expected?
Ans: \[{\text{Mn}}\] has half-filled 3d5 electrons and \[{\text{Zn}}\] has fully-filled 3d10 electrons which give them extra stability and they both resist to lose electrons and get reduced.
The \[{{\text{E}}^{{\circ }}}\] value also depends on the hydration enthalpy. More negative is the enthalpy of hydration, high is the \[{{\text{E}}^{{\circ }}}\] value. This is the reason behind\ [{\text{Ni}}\] having higher \[{{\text{E}}^{{\circ }}}\] value than expected.
34. Why is the first ionization enthalpy of \[Cr\] lower than that of \[Zn\]?
Ans: Cr(24)= [Ar]3d54s2
${{\text{Zn }}\left( {{\text{30}}} \right){\text{ = }}\left[ {{\text{Ar}}} \right]{\text{ 3}}{{\text{d}}^{\text{10}}}{\text{4}}{{\text{s}}^{\text{2}}}}$
The stability of orbitals according to the electrons filled is in order:
Fully filled > half-filled > partially filled
As \[{\text{3}}{{\text{d}}^{{\text{10}}}}\] is completely filled orbital of \[{\text{Zn}}\] is more stable than the \[{\text{3}}{{\text{d}}^{\text{5}}}\] half-filled orbital of \[{\text{Cr}}\] . It is comparatively easy to remove electron from \[{\text{3}}{{\text{d}}^{\text{5}}}\] than from \[{\text{3}}{{\text{d}}^{{\text{10}}}}\].
Therefore, the first ionization enthalpy of \[{\text{Cr}}\] is lower than that of \[{\text{Zn}}\].
35. Transition elements show high melting points. Why?
Ans: Transition elements have the ability to involve their d-electrons along with their s-electrons in the process of bond formation. This results in formation of strong metal-metal bonds which makes their melting point high.
36. When \[C{u^{2 + }}\] ion is treated with \[{\text{KI}}\], a white precipitate is formed. Explain the reaction with the help of a chemical equation.
Ans: ${\text{2C}}{{\text{u}}^{{\text{2 + }}}}{\text{ + 4}}{{\text{I}}^{\text{ - }}}{\text{ }} \to {\text{ C}}{{\text{u}}_{\text{2}}}{{\text{I}}_{\text{2}}}{\text{ + }}{{\text{I}}_{\text{2}}}$
When \[{\text{C}}{{\text{u}}^{{\text{2 + }}}}{\text{ }}\]reacts with potassium iodide white precipitate of \[{\text{C}}{{\text{u}}_{\text{2}}}{{\text{I}}_{\text{2}}}{\text{ }}\]is formed.
37. Out of \[C{u_2}C{l_2}\] and \[CuC{l_2}\], which is more stable and why?
Ans: Higher enthalpy of hydration provides extra stability to \[{\text{C}}{{\text{u}}^{{\text{2 + }}}}\] oxidation state than +1 oxidation state.
Enthalpy of hydration is described as the amount of energy released on dilution of one mole of gaseous ions.
38. When a brown compound of manganese (A) is treated with \[HCl\], it gives a gas
(B) The gas taken in excess, reacts with \[N{H_3}\] to give an explosive compound
(C). Identify compounds A, B and C.
Ans: The reactions are explained as:
${\text{Mn}}{{\text{O}}_{\text{2}}}{\text{ + 4HCl }} \to {\text{ MnC}}{{\text{l}}_{\text{2}}}{\text{ + C}}{{\text{l}}_{\text{2}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}}$
${\text{ (A) (B)}}$
${\text{3C}}{{\text{l}}_{\text{2}}}{\text{ + 2N}}{{\text{H}}_{\text{3}}}{\text{ }} \to {\text{ NC}}{{\text{l}}_{\text{3}}}{\text{ + 3HCl}}$
${\text{(excess) (C)}}$
39. Although fluorine is more electronegative than oxygen, the ability of oxygen to stabilize higher oxidation states exceeds that of fluorine. Why?
Ans: Fluorine - 1s22s22p5
${{\text{Oxygen - 1}}{{\text{s}}^{\text{2}}}{\text{ 2}}{{\text{s}}^{\text{2}}}{\text{ 2}}{{\text{p}}^{\text{6}}}{\text{ 3}}{{\text{s}}^{\text{2}}}{\text{ 3}}{{\text{p}}^{\text{6}}}} $
As we can infer from the electronic configuration, fluorine only has one unpaired electron in its 2p-orbital which can be involved in formation of only one bond and fluorine lacks d-orbital.
While oxygen has d-orbitals in its configuration which allow the oxygen to form multiple bonds. Therefore, oxygen has greater stability to stabilise its oxidation states than fluorine.
40. Although \[C{r^{3 + }}\] and \[C{o^{2 + }}\] ions have same number of unpaired electrons but the magnetic moment of Cr3+ is 3.87 BM and that of \[C{o^{2 + }}\] is 4.87 BM. Why?
Ans: Magnetic moment of an electron/dipole moment is caused by its intrinsic properties of spin and electric charge. It depends upon the number of unpaired electrons in its valence shell. The more the number of unpaired electrons, the greater will be the value of magnetic moment.
${\text{C}}{{\text{r}}^{{\text{3 + }}}}{\text{ = }}\left[ {{\text{Ar}}} \right]{\text{ 3}}{{\text{d}}^{\text{3}}}{\text{ = 3 unpaired electrons}}$
${\text{C}}{{\text{o}}^{{\text{2 + }}}}{\text{ = }}\left[ {{\text{Ar}}} \right]{\text{ 3}}{{\text{d}}^7}{\text{ = 3 unpaired electrons}}$
But as both the ions have the same no. of unpaired electrons, there’s one other factor called orbital contribution which also influences the magnetic moment. As, the three unpaired electronic configuration is symmetrical in \[{\text{C}}{{\text{r}}^{{\text{3 + }}}}\] this contributes zero orbital contribution but this is quite appreciable in case of \[{\text{C}}{{\text{o}}^{{\text{2 + }}}}\] and hence it has larger value of Magnetic moment than \[{\text{C}}{{\text{r}}^{{\text{3 + }}}}\]
41. Ionisation enthalpies of Ce, Pr and Nd are higher than Th, Pa and U. Why?
Ans: \[{\text{Ce, Pr and Nd}}\] belong to the lanthanide series whereas \[{\text{Th, Pa and U}}\] belong to the actinides family.
When electrons start accommodating the 4f and 5f orbitals, the 5f electrons penetrate less into the inner core. They are more effectively shielded nuclei in comparison to 4f-electrons in lanthanides. This leads to the fact that 5f-electrons experience reduced nuclear force of attraction and hence they have Lower ionisation enthalpies than lanthanoids.
42. Although \[{\text{Zr}}\] belongs to 4d and \[{\text{Hf}}\] belongs to the 5d transition series but it is quite difficult to separate them. Why?
Ans: As an effect of lanthanoid contraction, zirconium and hafnium have similar radius of 160pm and 159 pm respectively. Due to this similarity in their size, they show similar physical and chemical properties.
Lanthanoid contraction: Because the elements in Row 3 have 4f electrons. These electrons do not shield good, causing a greater nuclear charge. This greater nuclear charge has a greater pull on the electrons and result in the decrease in their size and atomic radii.
43. Although +3 oxidation state is the characteristic oxidation state of lanthanoids but cerium shows +4 oxidation state also. Why?
Ans: ${\text{Ce = 4}}{{\text{f}}^{\text{1}}}{\text{ 5}}{{\text{d}}^{\text{1}}}{\text{ 6}}{{\text{s}}^{\text{2}}}$
${\text{C}}{{\text{e}}^{{\text{3 + }}}}{\text{ = 4}}{{\text{f}}^1}{\text{ 5}}{{\text{d}}^0}{\text{ 6}}{{\text{s}}^0}$
As in \[{\text{C}}{{\text{e}}^{{\text{3 + }}}}\],
4f has only single electron. Cerium holds the capacity to lose this electron also and attain \[{\text{4}}{{\text{f}}^0}\] configuration which is more stable.
\[{\text{C}}{{\text{e}}^{{\text{4 + }}}}{\text{ = 4}}{{\text{f}}^0}{\text{ 5}}{{\text{d}}^0}{\text{ 6}}{{\text{s}}^0}\]
Hence it shows 4+ oxidation states.
44. Explain why does colour of \[KMn{O_4}\] disappear when oxalic acid is added to its solution in acidic medium?
Ans: \[{\text{2Mn}}{{\text{O}}_{\text{4}}}^{\text{ - }}{\text{ + 16}}{{\text{H}}^ + }{\text{ + 5}}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}^{{\text{2 - }}}{\text{ }} \to {\text{ 2M}}{{\text{n}}^{{\text{2 + }}}}{\text{ + 8}}{{\text{H}}_{\text{2}}}{\text{O + 10C}}{{\text{O}}_{\text{2}}}\]
\[{\text{KMn}}{{\text{O}}_{\text{4}}}\] oxidises the oxalic acid to CO2 and reduces itself to \[{\text{M}}{{\text{n}}^{{\text{2 + }}}}\] state. \[{\text{M}}{{\text{n}}^{{\text{2 + }}}}\]is colourless that’s why it seems that \[{\text{KMn}}{{\text{O}}_{\text{4}}}\] has been disappeared.
45. When orange solution containing \[C{r_2}{O_7}^{2 - }\] ion is treated with an alkali, a yellow solution is formed and when H+ ions are added to yellow solution, an orange solution is obtained. Explain why this happens?
Ans: This is due to the inter conversion of dichromate (orange) to chromate ion (yellow).
${\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}^{{\text{2 - }}}{\text{ }}\underset{{{{\text{H}}^{\text{ + }}}}}{\overset{{{\text{O}}{{\text{H}}^{\text{ - }}}}}{\longleftrightarrow}}{\text{ Cr}}{{\text{O}}_{\text{4}}}^{{\text{2 - }}}{\text{ }}$
${\text{(orange) (yellow)}}$
46. A solution of \[KMn{O_4}\] on reduction yields either a colourless solution or a brown precipitate or a green solution depending on pH of the solution. What different stages of the reduction do these represent and how are they carried out?
Ans: \[{\text{KMn}}{{\text{O}}_{\text{4}}}\] act as an oxidising agent however it’s activity as oxidising agent is influenced by the pH of the solution i.e. acidic, basic or neutral solution.
${{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}{\text{ + 2KOH }} \to {\text{ 2}}{{\text{K}}_{\text{2}}}{\text{Cr}}{{\text{O}}_{\text{4}}}{\text{ + }}{{\text{H}}_{\text{2}}}{\text{O}}$
${\text{ (Orange) (yellow)}}$
${\text{ 2}}{{\text{K}}_{\text{2}}}{\text{Cr}}{{\text{O}}_{\text{4}}}{\text{ + }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{ }} \to {\text{ }}{{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}{\text{ + }}{{\text{K}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{ + }}{{\text{H}}_{\text{2}}}{\text{O}}$
${\text{(yellow) (orange)}}$
47. The second and third rows of transition elements resemble each other much more than they resemble the first row. Explain why?
Ans: As an effect of lanthanide contraction, the second and third rows of transition elements resemble each other. For example, zirconium and hafnium have similar radius i.e. 160pm and 159pm respectively. Due to this similarity in their size, they show similar physical and chemical properties.
Lanthanoid contraction: Because the elements in Row 3 have 4f electrons. These electrons do not shield good, causing a greater nuclear charge. This greater nuclear charge has a greater pull on the electrons and result in the decrease in their size and atomic radii.
48. \[{\text{E}}\] of \[Cu\] is \[ + 0.34 V\] while that of \[{\text{Zn}}\] is\[ - 0.76 V\]. Explain.
Ans: The sum of sublimation energy and ionisation enthalpy to oxidise \[{\text{cu}}\left( {\text{s}} \right){\text{ to c}}{{\text{u}}^{{\text{2 + }}}}\]is so highly that it is not compensated by the hydration enthalpy of \[{\text{Cu}}\]. Due to this, the \[{\text{E}}\]of \[{\text{Cu}}\]is positive.
While in case if \[{\text{Zn}}\], the \[{\text{E}}\] value is negative or more negative than the expected value because when the electrons are removed from the 4s-orbital. \[{\text{Zn}}\] acquires a stable \[{\text{3}}{{\text{d}}^{{\text{10}}}}\] configuration state.
49. The halides of transition elements become more covalent with increasing oxidation state of the metal. Why?
Ans: As the positive charge of the ion increases or we can say that oxidation state of a transition element increases, its size decreases and as per Fajan’s rule, more the charge on the metal ion, more is its tendency to form covalent compounds because positively charged cation attracts the electron cloud strongly towards itself.
50. While filling up of electrons in the atomic orbitals, the 4s-orbital is filled before the 3d-orbital but reverse happens during the ionization of the atom. Explain why?
Ans: As per \[{\text{(n + l)}}\] rule, 4s has lower energy than 3d-orbital.
${{{3d - n + l = 3 + 2 = 5}}}$
${{\text{4s - n + l = 4 + 0 = 4}}}$
So, 4s are filled first.
After filling of electrons, 4s-orbital moves beyond 3d-orbital and 4s electrons are loosely held by the nucleus. Hence, electrons are removed first during the process of ionisation.
51. Reactivity of transition elements decreases almost regularly from \[{\text{Sc to Cu}}\]. Explain.
Ans: Ionisation enthalpies are the main factor that influence the reactivity of transition elements. Higher the ionisation enthalpy, lesser is the reacting of the transition element.
When we move along the period from \[{\text{Sc to Cu}}\], a regular increase in the ionisation enthalpy is observed which results in the almost regular decrease in the reactivity of elements.
MATCHING TYPE:
52. Match the catalysts given in Column I with the processes given in Column II.
Column I | Column II |
(i) Ni in the presence of hydrogen | (a) Ziegler Natta catalyst |
(ii)\[ C{u_2}C{l_2}\] | (b) Contact process |
(iii)\[{V_2}{O_5}\] | (c) Vegetable oil to ghee |
(iv) Finely divided iron | (d) Sandmeyer reaction |
(v)\[TiC{l_4} + Al{\left( {C{H_3}} \right)_3}\] | (e) Haber’s Process |
(f) Decomposition of \[KCl{O_3}\] |
Ans:
Column I | Column II |
(i) Ni in the presence of hydrogen | (c) Vegetable oil to ghee |
(ii)\[{\text{ C}}{{\text{u}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}{\text{}}\] | (d) Sandmeyer reaction |
(iii)\[{{\text{V}}_{\text{2}}}{{\text{O}}_{\text{5}}}{\text{}}\] | (b) Contact process |
(iv) Finely divided iron | (e) Haber’s Process |
(v)\[{\text{TiC}}{{\text{l}}_{\text{4}}}{\text{ + Al}}{\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{3}}}{\text{}}\] | (a) Zieglar Natta catalyst |
53. Match the compounds/elements given in Column I with uses given in Column II.
Column I (Compound/element) | Column II (Use) |
(i) Lanthanoid oxide | (a) Production of iron alloy |
(ii) Lanthanoid | (b) Television screen |
(iii) Misch metal | (c) Petroleum cracking |
(iv) Magnesium based alloy is constituent of | (d) Lanthanoid metal + iron |
(v) Mixed oxides of lanthanides are employed | (e) Bullets |
(f) In X-ray screen |
Ans:
Column I (Compound/element) | Column II (Use) |
(i) Lanthanide oxide | (b) Television screen |
(ii) Lanthanoid | (a) Production of iron alloy |
(iii) Misch metal | (d) Lanthanoid metal + iron |
(iv) Magnesium based alloy is constituent of | (e) Bullets |
(v) Mixed oxides of lanthanoids are employed | (c) Petroleum cracking |
Question 54. Match the properties given in Column I with the metals given in Column II.
Column I (Property) | Column II (Metal) |
(i) An element which can show +8 oxidation state | (a) Mn |
(ii) 3d block element that can show upto +7 oxidation state | (b) Cr |
(iii) 3d block element with highest melting point | (c) Os |
(d) Fe |
Ans:
Column I (Property) | Column II (Metal) |
(i) An element which can show +8 oxidation state | (c) Os |
(ii) 3d block element that can show upto +7 oxidation state | (a) Mn |
(iii) 3d block element with highest melting point | (b) Cr |
Question 55. Match the statements given in Column I with the oxidation states given in Column II.
Column I | Column II |
(i) Oxidation state of Mn in \[Mn{O_2}\] is | (a) + 2 |
(ii) Most stable oxidation state of Mn is | (b) + 3 |
(iii) Most stable oxidation state of Mn in oxides is | (c) + 4 |
(iv) Characteristic oxidation state of lanthanoids is | (d) + 5 |
(e) + 7 |
Ans:
Column I | Column II |
(i) Oxidation state of Mn in \[{\text{Mn}}{{\text{O}}_2}\]is | (c) + 4 |
(ii) Most stable oxidation state of Mn is | (a) + 2 |
(iii) Most stable oxidation state of Mn in oxides is | (e) + 7 |
(iv) Characteristic oxidation state of lanthanoids is | (b) + 3 |
56. Match the solutions given in Column I and the colours given in Column II.
Column I (Aqueous solution of salt) | Column II (Colour) |
(i)\[ FeS{O_4} .7{H_2}O\] | (a) Green |
(ii)\[NiC{l_2} .4{H_2}O\] | (b) Light pink |
(iii)\[MnC{l_2} .4{H_2}O\] | (c) Blue |
(iv)\[CoC{l_2} .6{H_2}O\] | (d) Pale green |
(v) \[C{u_2}C{l_2}\] | (e) Pink |
(f) Colourless |
Ans:
Column I (Aqueous solution of salt) | Column II (Colour) |
(i)\[{\text{ FeS}}{{\text{O}}_{\text{4}}}{\text{ }}{\text{.7}}{{\text{H}}_{\text{2}}}{\text{O}}\] | (d) Pale green |
(ii)\[{\text{NiC}}{{\text{l}}_{\text{2}}}{\text{ }}{\text{.4}}{{\text{H}}_{\text{2}}}{\text{O}}\] | (a) Green |
(iii)\[{\text{MnC}}{{\text{l}}_{\text{2}}}{\text{ }}{\text{.4}}{{\text{H}}_{\text{2}}}{\text{O}}\] | (b) Light pink |
(iv)\[{\text{CoC}}{{\text{l}}_{\text{2}}}{\text{ }}{\text{.6}}{{\text{H}}_{\text{2}}}{\text{O}}\] | (e) Pink |
(v) \[{\text{C}}{{\text{u}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}{\text{}}\] | (f) Colourless |
57. Match the property given in Column I with the element given in Column II.
Column I (Property) | Column II (Elements) |
(i) Lanthanoid which shows +4 oxidation state | (a) Pm |
(ii) Lanthanoid which can show +2 oxidation state | (b) Ce |
(iii) Radioactive lanthanoid | (c) Lu |
(iv) Lanthanoid which has \[4{f^7}\]electronic configuration in +3 oxidation state | (d) Eu |
(v) Lanthanoid which has \[4{f^{14}}\]electronic configuration in +3 oxidation state | (e) Gd |
(f) Dy |
Ans:
Column I (Property) | Column II (Elements) |
(i) Lanthanoid which shows +4 oxidation state | (b) Ce |
(ii) Lanthanoid which can show +2 oxidation state | (d) Eu |
(iii) Radioactive lanthanoid | (a) Pm |
(iv) Lanthanoid which has \[4{f^7}\]electronic configuration in +3 oxidation state | (e) Gd |
(v) Lanthanoid which has \[4{f^{14}}\]electronic configuration in +3 oxidation state | (c) Lu |
58. Match the properties given in Column I with the medals given in Column II.
Column I (Property) | Column II (Metal) |
(i) Element with highest second ionisation enthalpy | (a) Co |
(ii) Element with highest third ionisation Enthalpy | (b) Cr |
(iii) M in \[M {\left( {CO} \right)_6}\] is | (c) Cu |
(iv) Element with highest heat of atomisation | (d) Zn |
(e) Ni |
Ans:
Column I (Property) | Column II (Metal) |
(i) Element with highest second ionisation enthalpy | (c) Cu |
(ii) Element with highest third ionisation Enthalpy | (d) Zn |
(iii) M in \[{\text{M }}{\left( {{\text{CO}}} \right)_6}\]is | (b) Cr |
(iv) Element with highest heat of atomisation | (e) Ni |
ASSERTION AND REASON TYPE:
59. Assertion (A): \[C{u^{2 + }}\] iodide is not known.
Reason (R): \[C{u^{2 + }}\] oxidises I to iodine.
Ans: All halogens combine with copper to form copper halides except iodine. The reason behind this is that \[{\text{C}}{{\text{u}}^{{\text{2 + }}}}\] oxidises iodide (-1) to iodine (0). Therefore, \[{\text{C}}{{\text{u}}^{{\text{2 + }}}}\]iodide does not exist .Both the statements assertion and reason are correct and reason is the correct explanation of assertion.
60. Assertion (A): Separation of \[Zr\] and \[Hf\] is difficult.
Reason (R): Because \[Zr\] and \[Hf\] lie in the same group of the periodic table.
Ans: Although \[{\text{Zr}}\] and \[{\text{Hf}}\] lie in same group but As an effect of lanthanide contraction, zirconium and hafnium have similar radius of 160pm and 159 pm respectively. Due to this similarity in their size, they show similar physical and chemical properties. As a result, \[{\text{Zr}}\] and \[{\text{Hf}}\] are difficult to separate. Both the statements assertion and reason are correct but reason is not the correct explanation of assertion.
61. Assertion (A): Actinoids form relatively less stable complexes as compared to lanthanoids.
Reason (R): Actinoids can utilize their 5d orbitals along with 6d orbitals in bonding but lanthanoids do not use their 4f orbital for bonding.
Ans: Actinoids are more reactive than lanthanoids as they involve their 5d-orbital in the bond formation process while due to effectively shielded and greater nuclear charge 4f-orbitals are not available for bonding. Thus, lanthanoids comparatively form less complexes than actinoids.
Assertion is not true but reason is true.
62. Assertion (A): \[Cu\] cannot liberate hydrogen from acids .
Reason (R): because it has positive electrode potential.
Ans: \[{\text{Cu}}\] lies below hydrogen in electrochemical series and has positive electrode potential. hence cannot liberate hydrogen from acids.
Both the statements assertion and reason are correct and reason is the correct explanation of assertion.
63. Assertion (A): The highest oxidation state of osmium is +8.
Reason (R): Osmium is a 5d-block element.
Ans: Osmium has the capacity to expands its octet by utilising all the electrons from its 6s and 5d orbitals this results it to attain an oxidation state of +8.
Both the statements assertion and reason are correct but reason is not the correct explanation of assertion. The correct option is B.
LONG ANSWER TYPE:
64. Identify A to E and explain reaction involves:
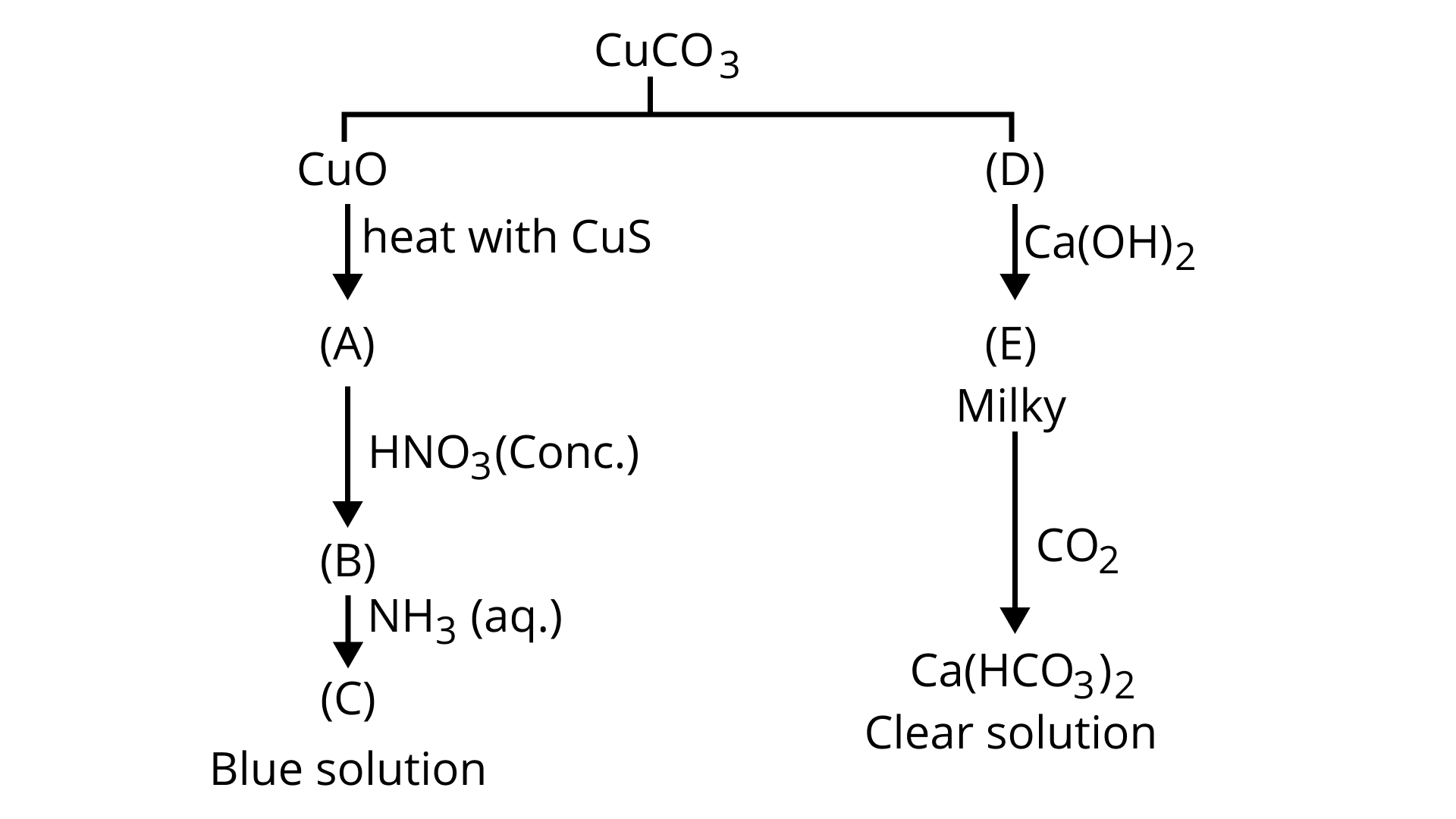
Ans:
${\text{CuC}}{{\text{O}}_{\text{3}}}{\text{ }}\xrightarrow{{{\text{heat}}}}{\text{ CuO + C}}{{\text{O}}_{\text{2}}}$
${\text{ (D)}}$
${\text{Ca(OH}}{{\text{)}}_{\text{2}}}{\text{ + C}}{{\text{O}}_{\text{2}}}{\text{ }} \to {\text{ CaC}}{{\text{O}}_3}{\text{ + }}{{\text{H}}_{\text{2}}}{\text{O}}$
${\text{ (E)}}$
${\text{CaC}}{{\text{O}}_3}{\text{ + C}}{{\text{O}}_{\text{2}}}{\text{ + }}{{\text{H}}_{\text{2}}}{\text{O }} \to {\text{ Ca(HC}}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}$
${\text{ clear sol}}{\text{.}}$
${\text{CuO + CuS }}\xrightarrow{{{\text{heat}}}}{\text{3Cu + S}}{{\text{O}}_{\text{2}}}{\text{ }}$
${\text{ (A) }}$
${\text{ Cu }} + 4{\text{HN}}{{\text{O}}_3}{\text{ }} \to {\text{ Cu(N}}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}{\text{ + 2N}}{{\text{O}}_{\text{2}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}}$
${\text{ (conc}}{\text{.) (B)}}$
${\text{Cu(N}}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}{\text{ + 4N}}{{\text{H}}_{\text{3}}}{\text{ }} \to {\text{ }}\left[ {{\text{Cu(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}} \right]{({\text{N}}{{\text{O}}_{\text{3}}}{\text{)}}_{\text{2}}}$
${\text{ (C) blue solution}}$
65. When a chromite ore (A) is fused with sodium carbonate in free excess of air and the product is dissolved in water, a yellow solution of compound (B) is obtained. After treatment of this yellow solution with sulphuric acid, compound (C) can be crystallized from the solution. When compound (C) is treated with \[KCl\], orange crystals of compound (D) crystallise out. Identify A to D and also explain the reactions.
Ans: ${\text{(A) = FeC}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{4}}}$
${\text{(B) = N}}{{\text{a}}_{\text{2}}}{\text{Cr}}{{\text{O}}_{\text{4}}}$
${\text{(C) = N}}{{\text{a}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_7}$
${\text{(D) = }}{{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}$
${\text{4FeCr}}{{\text{O}}_4}{\text{ + 8NaC}}{{\text{O}}_{\text{3}}}{\text{ + 7}}{{\text{O}}_{\text{2}}}{\text{ }}\xrightarrow{{}}{\text{ 8N}}{{\text{a}}_{\text{2}}}{\text{Cr}}{{\text{O}}_{\text{4}}}{\text{ + 2F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{ + 8C}}{{\text{O}}_{\text{2}}}$
${\text{ (A) (B)}}$
${\text{2NaCr}}{{\text{O}}_{\text{4}}}{\text{ + 2}}{{\text{H}}^{\text{ + }}}{\text{ }} \to {\text{ N}}{{\text{a}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_7}{\text{ + 2N}}{{\text{a}}^{\text{ + }}}{\text{ + }}{{\text{H}}_{\text{2}}}{\text{O}}$
${\text{N}}{{\text{a}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_7}{\text{ + KCl}} \to {\text{ }}{{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_7}{\text{ + 2NaCl }}$
${\text{ (C) (D) }}$
66. When an oxide of manganese (A) is fused with KOH in the presence of an oxidizing agent and dissolved in water, it gives a dark green solution of compound (B). Compound (B) disproportionate in neutral or acidic solution to give purple compound (C). An alkaline solution of compound (C) oxidises potassium iodide solution to a compound (D) and compound (A) is also formed. Identify compounds A to D and also explain the reactions involved.
Ans: ${\text{(A) = Mn}}{{\text{O}}_{\text{2}}}$
${\text{(B) = }}{{\text{K}}_{\text{2}}}{\text{Mn}}{{\text{O}}_{\text{4}}}$
${\text{(C) = KMn}}{{\text{O}}_{\text{4}}}{\text{ }}$
${\text{(D) = KI}}{{\text{O}}_{\text{3}}}$
${\text{2Mn}}{{\text{O}}_{\text{2}}}{\text{ + 4KOH + }}{{\text{O}}_{\text{2}}}{\text{ }}\xrightarrow{{}}{\text{ 2}}{{\text{K}}_{\text{2}}}{\text{Mn}}{{\text{O}}_{\text{4}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}}$
${\text{ (A) (B)}}$
${\text{3Mn}}{{\text{O}}_{\text{4}}}^{{\text{2 - }}}{\text{ + 4}}{{\text{H}}^{\text{ + }}}{\text{ }} \to {\text{ 2Mn}}{{\text{O}}_{\text{4}}}^{\text{ - }}{\text{ + Mn}}{{\text{O}}_{\text{2}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}}$
${\text{ (C)}}$
${\text{2Mn}}{{\text{O}}_{\text{4}}}^{{\text{2 - }}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O + KI}} \to {\text{ 2Mn}}{{\text{O}}_{\text{2}}}{\text{ + 2S}}{{\text{O}}_{\text{4}}}^{\text{ - }}{\text{ + KI}}{{\text{O}}_{\text{3}}}$
${\text{ (A) (D) }}$
67. On the basis of lanthanoid contraction, explain the following:
(a) Nature of bonding in \[L{a_2}{O_3}\] and \[L{u_2}{O_3}\].
Ans: As per fajan’s rule, smaller the size of ion, greater is its tendency to make covalent bonds as the positively charged cation strongly attract the negatively charged electron cloud.
On moving from \[{\text{La to Lu}}\] in the lanthanoid series, the atomic size decreases so the covalent character increases. Hence, \[{\text{L}}{{\text{a}}_{\text{2}}}{{\text{O}}_{\text{3}}}\]is ionic and \[{\text{L}}{{\text{u}}_{\text{2}}}{{\text{O}}_{\text{3}}}\]is covalent.
(b) Trends in the stability of oxo-salts of lanthanoids from \[La to Lu\].
Ans: The stability of Oxo salts is directly proportional to the size of atom, as we move from \[{\text{La to Lu}}\], the size of the atom decreases and hence the stability of oxo salts also decreases.
(c) Stability of the complexes of lanthanides.
Ans: As we move along the lanthanide series, the atomic size decreases. As a result, the charge/size ratio increases and the stability of the complexes also increases.
(d) Radii of 4d- and 5d-block elements.
Ans: As an effect of lanthanide contraction, zirconium and hafnium have similar radius of 160pm and 159 pm respectively. Due to this similarity in their size, they show similar physical and chemical properties. Lanthanoid contraction: Because the elements in Row 3 have 4f electrons. These electrons do not shield good, causing a greater nuclear charge. This greater nuclear charge has a greater pull on the electrons and result in the decrease in their size and atomic radii.
(e) Trends in acidic character of lanthanide oxides.
Ans: Acidic or basic character of oxides is related to the nature of oxides. As we move along the lanthanide series from La to Lu, the covalent character increases.
Therefore, their basic character decreases or acidic character increases.
68. (A) Answer the following questions:
(i) Which element of the first transition series has the highest second ionization enthalpy?
Ans: Out of all the elements of the first transition series copper has the highest second ionisation enthalpy.
Electronic configuration of Copper is: \[{\text{3}}{{\text{d}}^{{\text{10}}}}{\text{4}}{{\text{s}}^1}\]
After the Loss of first electron from the 4s copper acquires \[{\text{3d}}{{\text{ }}^{{\text{10}}}}\] configuration which is stable. Therefore, removal of second electron from the field 3-D orbital is very difficult and requires high amount of energy.
(ii) Which element of the first transition series has highest third ionization enthalpy?
Ans: Among the elements of first transition series zinc has the highest third ionisation enthalpy. Electronic configuration of zinc is: \[{\text{3}}{{\text{d}}^{{\text{10}}}}{\text{4}}{{\text{s}}^{\text{2}}}\]
After the loss of two electrons from 4s orbital, Z and +2 Ion acquires \[{\text{3d}}{{\text{ }}^{{\text{10}}}}\] fully filled configuration which is highly stable therefore removal of third electron from \[{\text{3d}}{{\text{ }}^{{\text{10}}}}\]orbital will be more difficult and requires a large amount of energy.
(iii) Which element of the first transition series has lowest enthalpy of atomization?
Ans: Zinc is the element of the first transition series that has the lowest enthalpy of atomisation. The electronic configuration of zinc is: \[{\text{3}}{{\text{d}}^{{\text{10}}}}{\text{4}}{{\text{s}}^{\text{2}}}\].This is because it has filled 3d-subshell and filled 4s-subshell so no unpaid electron is available for metallic bonding.
(b) Identify the metal and justify your answer:
\[\left( I \right) Carbonyl M{\left( {CO} \right)_5}\]
Ans: \[{\text{Fe}}{\left( {{\text{CO}}} \right)_{\text{5}}}\]as per EAN Rule.
EAN Rule: Effective atomic number (EAN), number that represents the total number of electrons surrounding the nucleus of a metal atom in a metal complex.
\[\left( {II} \right) M{O_3}F\]
Ans: The M in the given complex is in +7 O.S.
Manganese is the only element in the first transition series that shows +7 oxidation state, therefore this compound is \[{\text{Mn}}{{\text{O}}_{\text{3}}}{\text{F}}\].
69. Mention the type of compounds formed when small atoms like \[H, C and N\] get trapped inside the crystal lattice of transition metals. Also give physical and chemical characteristics of these compounds.
Ans: Interstitial compounds are formed when small atoms like \[{\text{H, C and N}}\] get trapped inside the crystal lattice of transition metals.
Characteristics:
They are hard and rigid in nature having high melting points.
Like pure metals only, they are conductors. They can gain chemical inertness.
70. (A) Transition metals can act as catalysts because these can change their oxidation state.
How does \[Fe\left( {III} \right)\]catalyse the reaction between iodide and persulphate ions?
Ans: ${\text{2}}{{\text{I}}^{\text{ - }}}{\text{ + }}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{8}}}^{{\text{2 - }}}{\text{ }}\xrightarrow{{{\text{Fe(III)}}}}{\text{ }}{{\text{I}}_{\text{2}}}{\text{ + 2S}}{{\text{O}}_{\text{4}}}^{{\text{2 - }}}$
${\text{ role of fe ions:}}$
${\text{2F}}{{\text{e}}^{{\text{3 + }}}}{\text{ + 2I - }} \to {\text{ 2F}}{{\text{e}}^{{\text{2 + }}}}{\text{ + }}{{\text{I}}_{\text{2}}}$
${\text{2F}}{{\text{e}}^{{\text{2 + }}}}{\text{ + }}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{8}}}^{{\text{2 - }}} \to {\text{ 2F}}{{\text{e}}^{{\text{3 + }}}}{\text{ + 2S}}{{\text{O}}_{\text{4}}}^{{\text{2 - }}}$
(B) Mention any three processes where transition metals act as catalysts.
Ans: 1. Fine powdered state of Nickel is used in the hydrogenation of oils into fats.
2. Iron is used in the formation of ammonia in Haber’s process.
3. In contact process in manufacture of \[{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\], vanadium is used in its oxide form- \[{{\text{V}}_{\text{2}}}{{\text{O}}_{\text{5}}}\].
71. A violet compound of manganese (A) decomposes on heating to liberate oxygen and compounds (B) and (C) of manganese are formed. Compound (C) reacts with \[KOH\] in the presence of potassium nitrate to give compound (B). On heating compound (C) with conc. \[{H_2}S{O_4}\]and \[NaCl\], chlorine gas is liberated and a compound (D) of manganese along with other products is formed. Identify compounds A to D and also explain the reactions involved.
Ans: ${\text{KMn}}{{\text{O}}_{\text{4}}}{\text{ }}\xrightarrow{\vartriangle }{\text{ }}{{\text{K}}_{\text{2}}}{\text{Mn}}{{\text{O}}_{\text{4}}}{\text{ + Mn}}{{\text{O}}_{\text{2}}}{\text{ + }}{{\text{O}}_{\text{2}}}$
${\text{ (A) (B) (C)}}$
${\text{Mn}}{{\text{O}}_{\text{2}}}{\text{ + KOH }} \to {\text{ 2}}{{\text{K}}_{\text{2}}}{\text{Mn}}{{\text{O}}_{\text{4}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}}$
${\text{Mn}}{{\text{O}}_{\text{2}}}{\text{ + 4NaCl + 4}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\xrightarrow{{}}{\text{ MnC}}{{\text{l}}_{\text{2}}}{\text{ + 2NaHS}}{{\text{O}}_{\text{4}}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O + C}}{{\text{l}}_{\text{2}}}$
${\text{ (D)}}$
${\text{(A) = KMn}}{{\text{O}}_{\text{4}}}$
${\text{(B) = }}{{\text{K}}_{\text{2}}}{\text{Mn}}{{\text{O}}_{\text{4}}}$
${\text{(C) = Mn}}{{\text{O}}_{\text{2}}}$
${\text{(D) = MnC}}{{\text{l}}_{\text{2}}}$
Benefits of Using the Solutions for Class 12 NCERT Exemplar for Chapter 8 The d and f Block Elements
By using the solutions for Class 12 NCERT Exemplar for Chapter 8 The d and f Block elements, students can have the following advantages:-
Students can solve questions that can directly appear in their Exams
Students get to understand how answers are supposed to be written
Students can use the solutions for their mock tests
Students can distinguish between the most important and the less important topics from the Chapter.
Conclusion
NCERT Exemplar can help students in scoring well in their Exams. With a more Exam focussed approach in their revision, students can ensure the maximum marks for themselves. By using and solving this free PDF document for students of Class 12 covering the solutions to the book Exercises from NCERT Exemplar for Chapter 8- The d and f Block elements, success is inevitable!
FAQs on NCERT Exemplar for Class 12 Chemistry Chapter-8 (Book Solutions)
1. Can I expect direct questions from Chapter 7- The d and f Block elements in my competitive Exams like JEE and NEET?
Yes, The d and f Block elements is an easy-to-understand Chapter that can guarantee you some really easy marks from direct questions too. Inorganic Chemistry is a great way for students to make their scores go higher. By going through Exercises in the Class 12 NCERT textbook and even NCERT Exemplar, students can get good marks at this simple topic. Do not neglect this Chapter at any cost because it is an important Chapter for the board Exams and the competitive Exams. You can understand all concepts to score high marks in this Chapter.
2. What should be my strategy to study The d and f Block Elements for NEET?
NEET requires a good understanding and clear basics of any topic for the students to get good marks. The MCQ pattern may be easy for a lot of people but there is also a big portion of the student population who make a lot of silly mistakes in MCQs. The strategy to study this Chapter shall remain the same just like every other Chapter. Read the Chapter from your NCERT textbook, make your notes and then solve as many MCQs as possible.
Vedantu is a leading website that offers performance-enhancing resources for students of Class 12 preparing for NEET. Here is a video that covers some of the most important MCQs from the Chapter The d and f Block Elements for NEET.
3. Is it important to study from NCERT Exemplar for Class 12?
NCERT Exemplar is a great book that has some really good questions. These questions are not only of a higher level compared to the NCERT textbook but are also very helpful for Competitive Exams.
Therefore, it is recommended to get into the habit of solving NCERT Exemplar so that students can diversify their knowledge and increase their chances of scoring more. It is an excellent study material for the students of Class 12 as they can get a number of questions to solve and practice for the final Exams.
4. Is it possible to score full marks in The d and f Block Elements in my Class 12 Board Examinations?
It is definitely possible to score full marks in your Class 12 board Examinations. Board Examinations require a good understanding of how answers are written. Students must be aware of recurring patterns and trends from the Exam over the past several years. Solving previous year’s question papers can be very helpful in this scenario.
With Vedantu, you can easily download the previous year’s question papers so that you do not have to look anywhere else. Click here to get the previous year’s question papers so that you score the best in your CBSE Board Examinations!
5. Is The d and f Block Elements a difficult Chapter?
Thankfully, no. The d and f Block elements are not a difficult Chapter. It is very easy to understand and comprehend. This can be a very helpful Chapter if students want to score easy marks from a Chapter. No Chapter from Inorganic Chemistry must be skipped. Inorganic Chemistry is a good way to boost your score in Chemistry in the final Exams.
The d and f Block elements is one such interesting and simple Chapter that guarantees high returns in your Exams!

























