Download Free PDF of Class 8 Science NCERT Exemplar Solutions Chapter 14 Chemical Effects of Electric Current available on Vedantu
FAQs on NCERT Exemplar for Class 8 Science Solutions Chapter 14 Chemical Effects of Electric Current
1. What will I learn from Chapter 14 of NCERT Exemplar Solutions for Class 8 Science?
This chapter deals with the chemical effects of electric current, electroplating, good conductors, LED, and conductors. All The topics explained in this chapter are very important for the examination. A detailed explanation of some topics like the liquid conduct of electricity, chemical effects of electric current, etc. is given to help the students better understand the topic. After going through this chapter you will be able to differentiate between the good conductors of electricity and poor conductors of electricity which is a very important topic for the exam.
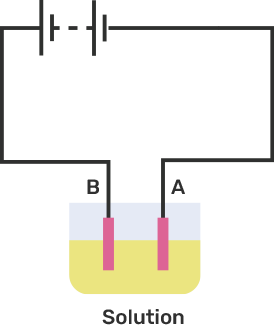
2. An electric current is passed through a conducting solution. What are the possible observations according to Chapter 14 of NCERT Exemplar Solutions for Class 8 Science?
Class 8 Science NCERT Exemplar Solutions Chapter 14 Chemical Effects of Electric Current provides yo answers to all the questions you can easily go through it and know the right answer. Also, students get to know the pattern in which they should write an answer according to CBSE guidelines and score well in exams.
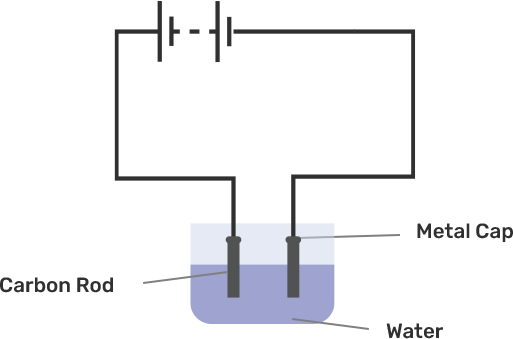
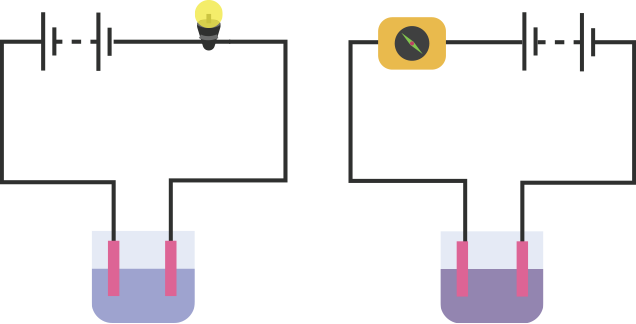
An electric current is passed through a conducting solution. The possible observations will be
Bubbles of gas can be formed on the electrodes.
Deposits of metal might be seen on electrodes.
Change in the colour of the solution might take place
The solution can get heated.
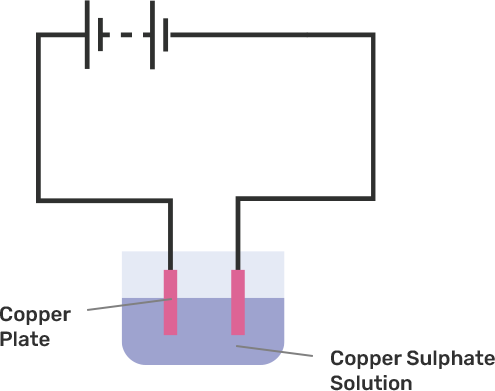
3. Explain electroplating covered in Class 8 Science NCERT Exemplar Solutions Chapter 14 Chemical Effects of Electric Current?
The process by which the plating of metal to others is done to prevent corrosion of metal or for decoration purposes is known as Electroplating. In this process, electric current is used to reduce dissolved metal cations and to develop a lean coherent metal coating on the electrode. Students can get references from Class 8 Science NCERT Exemplar Solutions Chapter 14 Chemical Effects of Electric Current which is prepared by the experts at Vedantu, it enables you to understand the concept easily.
4. Why is Vedantu the best platform to provide Class 8 Science NCERT Exemplar Solutions Chapter 14 Chemical Effects of Electric Current?
Vedantu is India’s #1 online learning portal that has excellent teachers that believe that teaching is not just a profession, it is a commitment and a matter of pride and great responsibility. Vedantu has the best technologies to provide the best study experience to the students and help them to grow in a better technical way. Moving with time is a necessity and therefore Vedantu provides you with the study material that is updated according to the latest syllabus and the latest guideline of CBSE. This will help you to get top rank in your Class 8 final examination.
5. What type of questions are asked in Class 8 Science NCERT Exemplar Solutions Chapter 14?
Class 8 Science NCERT Exemplar Solutions Chapter 14 Chemical Effects of Electric Current is prepared to help students to get knowledge about the type and pattern of questions that can be asked in the examinations. The different patterns of questions asked in exams are:
Multiple-choice questions
Very short answer questions
Short answer question
Long answer questions.
You can prepare them all with Class 8 Science NCERT Exemplar Solutions prepared by Vedantu. it provides you with 100% accurate answers and is the most reliable book.