Class 9 Science Chapter 2 - Is Matter Around Us Pure - Free PDF download of NCERT Exemplar
Free PDF download of NCERT Exemplar for Class 9 Science Chapter 2 - Is Matter Around Us Pure solved by expert Science teachers on Vedantu as per NCERT (CBSE) Book guidelines. All Chapter 2 - Is Matter Around Us Pure exercise questions with solutions to help you to revise the complete syllabus and score more marks in your examinations. The NCERT Solutions are always beneficial in your exam preparation and revision. Download NCERT Solutions for Class 9 Maths from Vedantu, which are curated by master teachers. Science Students who are looking for Class 9 Science NCERT Solutions will also find the Solutions curated by our Master Teachers really Helpful.
NCERT Exemplar Solutions for Class 9 Science (Chemistry) Chapter 2 - Is Matter around us Pure
Multiple Choice Questions
1. Which of the following statements are true for pure substances?
i. Pure substances contain only one kind of particle.
ii. Pure substances may be compounds or mixtures.
iii. Pure substances have the same composition throughout.
iv. Pure substances can be exemplified by all elements other than nickel.
(i) and (ii)
(i) and (iii)
(iii) and (iv)
(ii) and (iii)
Ans: (b) (i) and (iii)
The pure substances include only one kind of particle and have the same composition throughout. Hence the correct answer is option (b).
2. Rusting of an article made up of iron is called:-
a. corrosion and it is a physical as well as chemical change.
b. dissolution and it is a physical change.
c. corrosion and it is a chemical change.
d. dissolution and it is a chemical change.
Ans: (c) corrosion and it is a chemical change.
The rusting of iron is a chemical change. It results in the corrosion of iron.
Hence the correct answer is option (c).
3. A mixture of sulphur and carbon disulphide is:-
heterogeneous and shows Tyndall effect.
homogeneous and shows Tyndall effect.
heterogeneous and does not show Tyndall effect.
homogeneous and does not show Tyndall effect.
Ans: (d) homogeneous and does not show Tyndall effect.
The given mixture of the sulphur and the carbon disulphide is a homogeneous mixture. The carbon disulphide is used as a solvent for the sulphur. The Tyndall effect is caused by the scattering of light by insoluble particles present in a solution. The colloids and suspensions, which are heterogeneous mixtures, contain insoluble particles, and thus show Tyndall effect. Hence the correct answer is option (d).
4. Tincture of iodine has antiseptic properties. This solution is made by dissolving:-
iodine in potassium iodide.
iodine in vaseline.
iodine in water.
iodine in alcohol.
Ans: (d) iodine in alcohol
A solution of "Tincture of Iodine" is made by dissolving 2 - 7% iodine into the alcohol.The tincture solutions are characterised by the presence of alcohol because the alcohol is a good solvent. The iodine does not dissolve in water. The tincture of Iodine is also used as an antiseptic. Hence the correct answer is option (d).
5. Which of the following are homogeneous in nature?
(i) ice (ii) wood (iii) soil (iv) air
(i) and (iii)
(ii) and (iv)
(i) and (iv)
(iii) and (iv)
Ans: (c) (i) and (iv)
The ice and air are homogeneous mixtures. The soil is a heterogeneous mixture and the wood also has a heterogeneous composition. Hence the correct answer is option (c).
6. Which of the following are physical changes?
i. Melting of iron metal
ii. Rusting of iron
iii. Bending of an iron rod
iv. Drawing a wire of iron metal
(i), (ii) and (iii)
(i), (ii) and (iv)
(i), (iii) and (iv)
(ii), (iii) and (iv)
Ans: (c) (i), (iii) and (iv)
The rusting of iron is a chemical change. A new substance like iron oxide is formed in case rusting of iron takes place. In the other given options, no new substance is formed, and they are physical changes. Hence the correct answer is option (c).
7. Which of the following are chemical changes?
i. Decaying of wood
ii. Burning of wood
iii. Sawing of wood
iv. Hammering of a nail into a piece of wood
(i) and (ii)
(ii) and (iii)
(iii) and (iv)
(i) and (iv)
Ans: (a) (i) and (ii)
The burning and decay result in the formation of new substances and they cannot be reversed. Therefore, they are under chemical changes. The sawing of wood and hammering of a nail into a piece of wood are under physical changes. Hence the correct answer is option (a).
8. Two substances, A and B, were made to react to form a third substance A2B according to the following reaction: 2A + B → A2B. Which of the following statements concerning this reaction are incorrect?
i. The product A2B shows the properties of substances A and B.
ii. The product will always have a fixed composition.
iii. The product so formed cannot be classified as a compound.
iv. The product so formed is an element.
(i), (ii) and (iii),
(ii), (iii) and (iv)
(i), (iii) and (iv)
(ii), (iii) and (iv)
Ans: (c) (i), (iii) and (iv)
The properties of the compound A2B that is the product formed as a result of the reaction are different from the properties of its constituent elements - "A" and "B". The A2B will always have "A" and "B" in the fixed composition by the mass. Hence the correct answer is option (c).
9. Two chemical species X and Y combine together to form a product P which contains both X and Y. X + Y → P
X and Y cannot be broken down into simpler substances by simple chemical reactions.
Which of the following concerning the species X, Y and P are correct?
i. P is a compound.
ii. X and Y are compounds.
iii. X and Y are elements.
iv. P has a fixed composition.
(i), (ii) and (iii),
(i), (ii) and (iv)
(ii), (iii) and (iv)
(i), (iii) and (iv)
Ans: (d) (i), (iii) and (iv)
Since "X" and "Y" cannot be broken down further by the simple chemical reactions are not compounds, they are elements. Therefore (iii) is correct and (ii) is incorrect. The product "P" is a compound of elements "X" and "Y". Therefore (i) is correct. The "P" compound will always have a fixed composition of the constituent elements "X" and "Y". Therefore (iv) is correct. Hence the correct answer is option (d).
Short Answer Questions
10.Suggest separation technique(s) one would need to employ to separate the following mixtures:
a. Mercury and water
Ans: By separating the funnel. The mercury and water are immiscible liquids. The mercury is more dense than the water and can be separated by using a separating funnel.
b. Potassium chloride and ammonium chloride
Ans: By sublimation method. The ammonium chloride is a sublimating compound and can be separated from the mixture through the sublimation method
c. Common salt, water and sand
Ans: By filtration of sand followed by the evaporation of water OR by centrifugation to separate sand followed by the evaporation/distillation of the water.
d. Kerosene oil, water and salt
Ans: By separating funnel to separate kerosene oil followed by the evaporation / distillation of the water.
11. Which of the tubes in Fig. 2.1 (a) and (b) will be more effective as a condenser in the distillation apparatus?
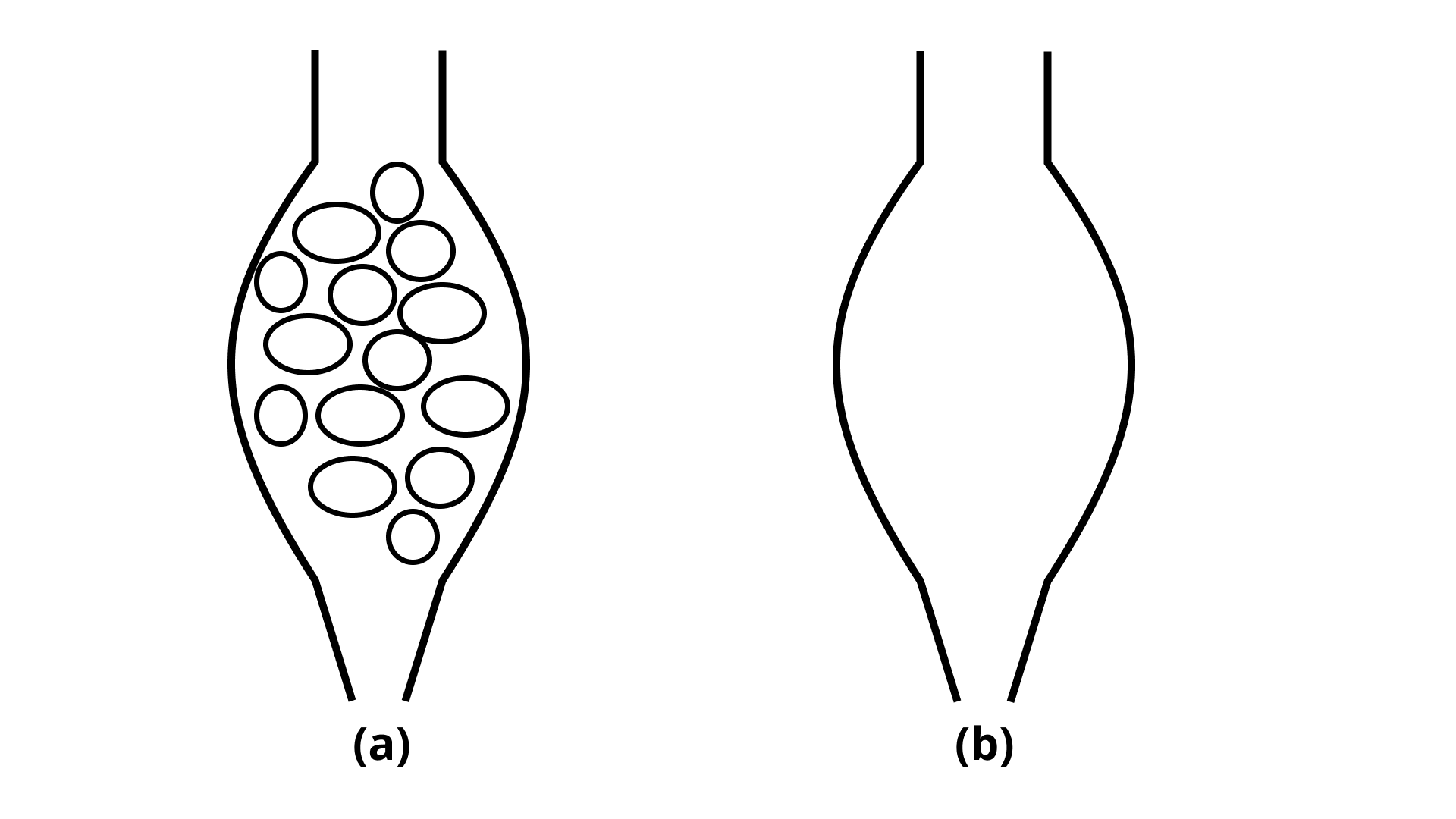
Ans: The tube (a) will be more effective than tube (b) as a condenser in the distillation apparatus. The presence of marbles increases the surface area that comes in contact with the vapours. This allows more time for the condensation of vapours and therefore the first column would be more effective than the second column without the marbles.
12. Salt can be recovered from its solution by evaporation. Suggest some other technique for the same?
Ans: The salt can also be recovered from its solution by the crystallization technique.
13. The ‘sea-water’ can be classified as a homogeneous as well as heterogeneous mixture. Comment.
Ans: The sample of sea-water collected from the surface of sea: The sea water can be considered as a homogeneous mixture when it has salts and water only.
The sample of the sea-water collected from the deep sea: The sea water will be a heterogeneous mixture when the sample is collected from the deeper layers and it contains salts, water, mud, decayed plants, etc.
14. While diluting a solution of salt in water, a student by mistake added acetone (boiling point 56 °C). What technique can be employed to get back the acetone? Justify your choice.
Ans: The acetone can be separated from the mixture of the salt solution and water by the distillation method. There is a considerable difference in the boiling point of the acetone which is 56 °C and the boiling point of the water which is 100 °C. Therefore when the solution is heated, the acetone will evaporate before the water does and it can be collected after the condensation.
15. What would you observe when:
a. a saturated solution of potassium chloride prepared at 60 °C is allowed to cool to room temperature.
Ans: The solid potassium chloride will separate out from the saturated solution when the temperature of the solution reduces from the 60°C to the room temperature. A change in the temperature affects the solubility of the solid solute.
b. an aqueous sugar solution is heated to dryness.
Ans: When the aqueous sugar solution is heated, the water will initially evaporate. When the solution is heated to the dryness, the sugar will get charred.
c. a mixture of iron filings and sulphur powder is heated strongly.
Ans: The iron will combine chemically with the sulphur when heated strongly and iron sulphide (FeS) will be formed.
16. Explain why particles of a colloidal solution do not settle down when left undisturbed, while in the case of a suspension they do.
Ans: In a suspension, the size of the particles is relatively larger than that of a colloidal solution. Also, the molecular interaction in a suspension is not strong enough to keep the particles suspended. Therefore the particles settle down when the suspension is left undisturbed for some time. On the other hand, the molecular interaction in a colloidal solution like milk is strong enough and does not allow the particles to settle down.
17. Smoke and fog both are aerosols. In what way are they different?
Ans: Both the - fog and smoke - have gas as the dispersion medium that is the continuous phase. The difference lies in the dispersed phase that is the suspended phase. The dispersed phase in the fog is liquid like water droplets whereas the dispersed phase in the smoke is solid like the particulate matter.
18. Classify the following as physical or chemical properties:
a. The composition of a sample of steel is: 98% iron, 1.5% carbon and 0.5% other elements.
Ans: The physical property of iron: The steel is an alloy of iron with about one percent carbon.
b. Zinc dissolves in hydrochloric acid with the evolution of hydrogen gas.
Ans: The chemical property of zinc: The zinc is a reactive metal. It displaces hydrogen from the hydrochloric acid and the formation of zinc chloride takes place.
c. Metallic sodium is soft enough to be cut with a knife.
Ans: The physical property of sodium: The sodium is a soft metal.
d. Most metal oxides form alkalis on interacting with water.
Ans: The chemical property of metallic oxides: The metallic oxides react with water and form the alkalies.
19. The teacher instructed three students ‘A’, ‘B’ and ‘C’ respectively to prepare a 50% (mass by volume) solution of sodium hydroxide (NaOH).
‘A’ dissolved 50 g of NaOH in 100 ml of water, ‘B’ dissolved 50 g of NaOH in 100 g of water while ‘C’ dissolved 50 g of NaOH in water to make 100 ml of solution. Which one of them has made the desired solution and why?
Ans: Student ' C ' has made the desired solution.
Mass by volume % = (Mass of solute / Volume of solution) X Then, 100 =(50g / 100ml)×100
= 50%
mass by volume
20. Name the process associated with the following:
a. Dry ice is kept at room temperature and at one atmospheric pressure.
Ans: By the sublimation process.(The dry ice or the solid carbon dioxide sublimes at lower temperatures into the large volumes of CO2 gas and is dangerous to handle. It can also cause burns by freezing).
b. A drop of ink placed on the surface of water contained in a glass spreads throughout the water.
Ans: By the diffusion of ink in water.
c. A potassium permanganate crystal is in a beaker and water is poured into the beaker with stirring.
Ans: By the dissolution/diffusion of the potassium permanganate.
d. A acetone bottle is left open and the bottle becomes empty.
Ans: By the evaporation and diffusion of the acetone.
e. Milk is churned to separate cream from it.
Ans: By the centrifugation of the milk.
f. Settling of sand when a mixture of sand and water is left undisturbed for some time.
Ans: By the sedimentation of sand.
g. Fine beam of light entering through a small hole in a dark room, illuminates the particles in its paths.
Ans: By the scattering of light (Tyndall effect).
21. You are given two samples of water labelled as ‘A’ and ‘B’. Sample ‘A’ boils at 100°C and sample ‘B’ boils at 102 °C. Which sample of water will not freeze at 0°C? Comment.
Ans: The sample ‘B’ will not freeze at 0°C because it may contain some impurities. At 1 atm, the boiling point of the pure water is 100°C and the freezing point of the pure water is 0°C.
22. What are the favourable qualities given to gold when it is alloyed with copper or silver for the purpose of making ornaments?
Ans: The pure gold that is 24-carat gold is very soft. It is alloyed with the silver or copper to impart strength while making the ornaments. An alloy that contains 20 parts of the gold and 4 parts of the silver is called a 20-carat gold.
23. An element is sonorous and highly ductile. Under which category would you classify this element? What other characteristics do you expect the element to possess?
Ans: The metals are sonorous and highly ductile; therefore this element can be classified as a metal. The other characteristics expected to be possessed by this element are – lustre, malleability, heat and the electrical conductivity.
24. Give an example each for the mixture having the following characteristics. Suggest a suitable method to separate the components of these mixtures:
a. A volatile and a non-volatile component.
Ans: The ammonium chloride and the sodium chloride. The mixture can be separated by the sublimation process of the ammonium chloride.
b. Two volatile components with appreciable difference in boiling points.
Ans: The acetone and water. The mixture can be separated by the evaporation process or by the distillation process. The boiling point of the acetone is 56 °C. The boiling point of water is 100 °C. The difference in the boiling points is 44 °C.
c. Two immiscible liquids.
Ans: The kerosene and water. The mixture can be separated with the help of a separating funnel.
d. One of the components changes directly from solid to gaseous state.
Ans: The potassium chloride and the ammonium chloride. The mixture can be separated by the sublimation process of the ammonium chloride.
e. Two or more coloured constituents are soluble in some solvent.
Ans: The plant pigments. The mixture of the coloured constituents can be separated by the chromatography method.
25. Fill in the blanks:
a. A colloid is a mixture and its components can be separated by the technique known as .
Ans: heterogeneous, centrifugation.
b. Ice, water and water vapour look different and display different properties but they are the same.
Ans: physical, chemically.
c. A mixture of chloroform and water taken in a separating funnel is mixed and left undisturbed for some time. The upper layer in the separating funnel will be of and the lower layer will be that of .
Ans: Water, chloroform. (The density of water is less than that of chloroform.)
d. A mixture of two or more miscible liquids, for which the difference in the boiling points is less than 25 K can be separated by the process called .
Ans: fractional distillation.
e. When light is passed through water containing a few drops of milk, it shows a bluish tinge. This is due to the of light by milk and the phenomenon is called . This indicates that milk is a solution.
Ans: scattering, Tyndall effect, colloidal
26. Sucrose (sugar) crystals obtained from sugarcane and beetroot are mixed together.
Will it be a pure substance or a mixture? Give reasons for the same.
Ans: It will be a pure substance compound since the chemical composition of sugar crystals will be the same whether obtained from the sugarcane or from beetroot.
27. Give some examples of Tyndall effects observed in your surroundings?
Ans: Following are some of the examples of the Tyndall effect:-
The sunlight entering a room through a ventilation near the ceiling.
The beam of light coming inside a forest through a canopy of trees.
28. Can we separate alcohol dissolved in water by using a separating funnel? If yes, then describe the procedure. If not, explain.
Ans: The water that is a universal solvent and the alcohol which is an organic solvent, are miscible liquids. Therefore, a separating funnel cannot be used to separate them from their mixture.
29. On heating calcium carbonate gets converted into calcium oxide and carbon dioxide.
a. Is this a physical or a chemical change?
Ans: The chemical change that is the decomposition of the calcium carbonate.
CaCO3 → CaO + CO2
b. Can you prepare one acidic and one basic solution by using the products formed in the above process? If so, write the chemical equation involved.
Ans: The acidic and the basic solutions can be prepared by dissolving the products of the above process in water.
The calcium oxide will form a basic solution.
CaO + H2O → Ca(OH)2
(Basic solution)
The carbon dioxide will form an acidic solution.
CO2 + H2O → H2CO3
(Acidic solution)
30. Non-metals are usually poor conductors of heat and electricity. They are non lustrous, non-sonorous, non-malleable and are coloured.
a. Name a lustrous non-metal.
Ans: Iodine
b. Name a non-metal which exists as a liquid at room temperature.
Ans: Bromine
c. The allotropic form of a non-metal is a good conductor of electricity. Name the allotrope.
Ans: Graphite
d. Name a non-metal which is known to form the largest number of compounds.
Ans: Carbon
e. Name a non-metal other than carbon which shows allotropy.
Ans: Sulphur, phosphorus
f. Name a non-metal which is required for combustion.
Ans: Oxygen
31. Classify the substances given in Fig. 2.2 into elements and compounds:
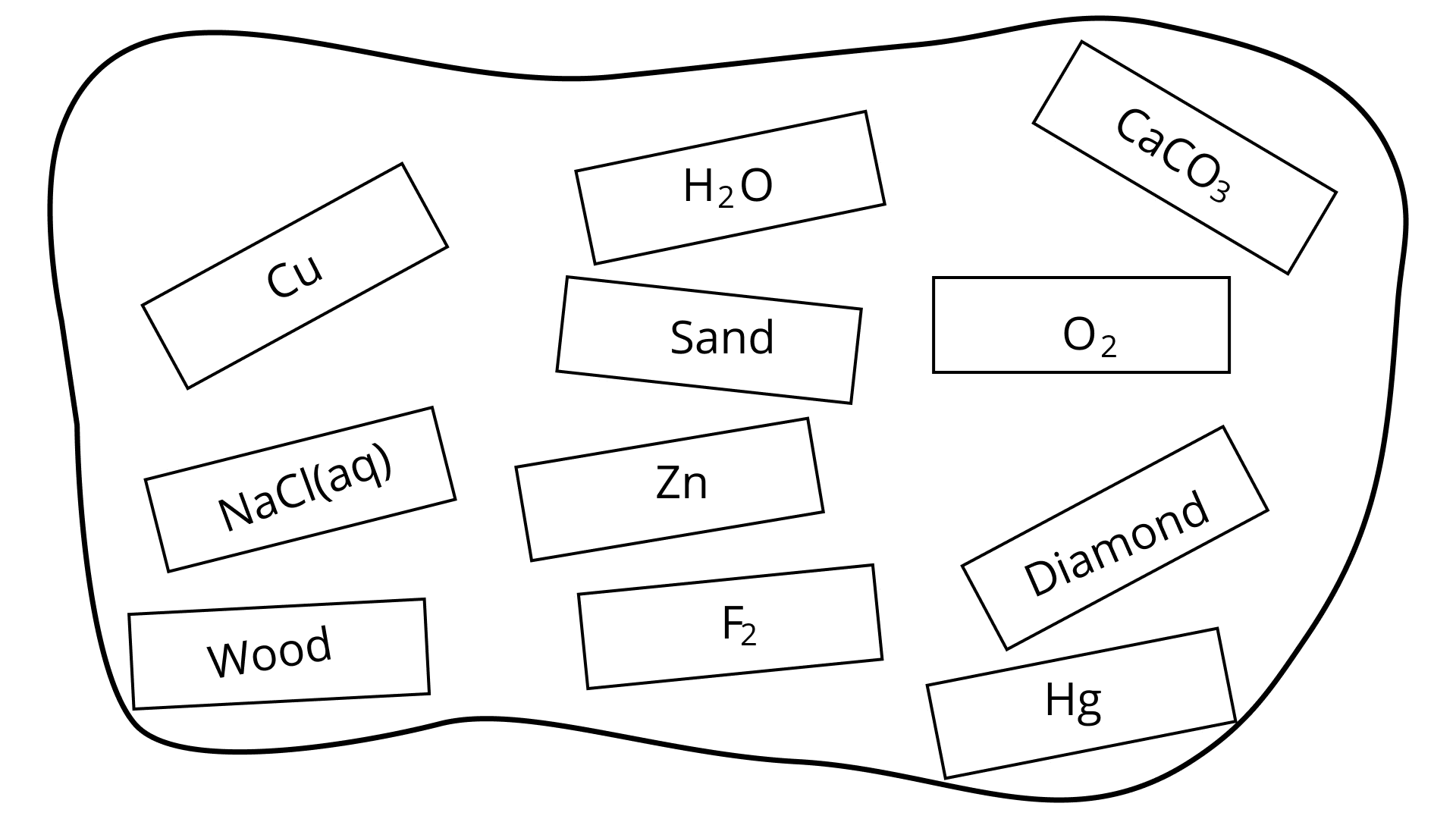
Ans: Elements:
H2O, CaCO3 .
Cu, Zn,O2 , F2 , Hg , Diamond Compounds: NaCl(aq) , Wood, Sand,
32. Which of the following are not compounds?
(a) Chlorine gas (b) Potassium chloride (c) Iron (d) Iron sulphide
(e) Aluminium (f) Iodine (g) Carbon (h) Carbon monoxide
(i) Sulphur powder
Ans: The following are not compounds: Chlorine gas, Iron, Aluminium, Iodine, Carbon, Sulphur powder.
Long Answer Questions
33. Fractional distillation is suitable for separation of miscible liquids with a boiling point difference of about 25 K or less. What part of fractional distillation apparatus makes it efficient and possess an advantage over a simple distillation process? Explain using a diagram.
Ans: Fractional distillation of miscible liquids
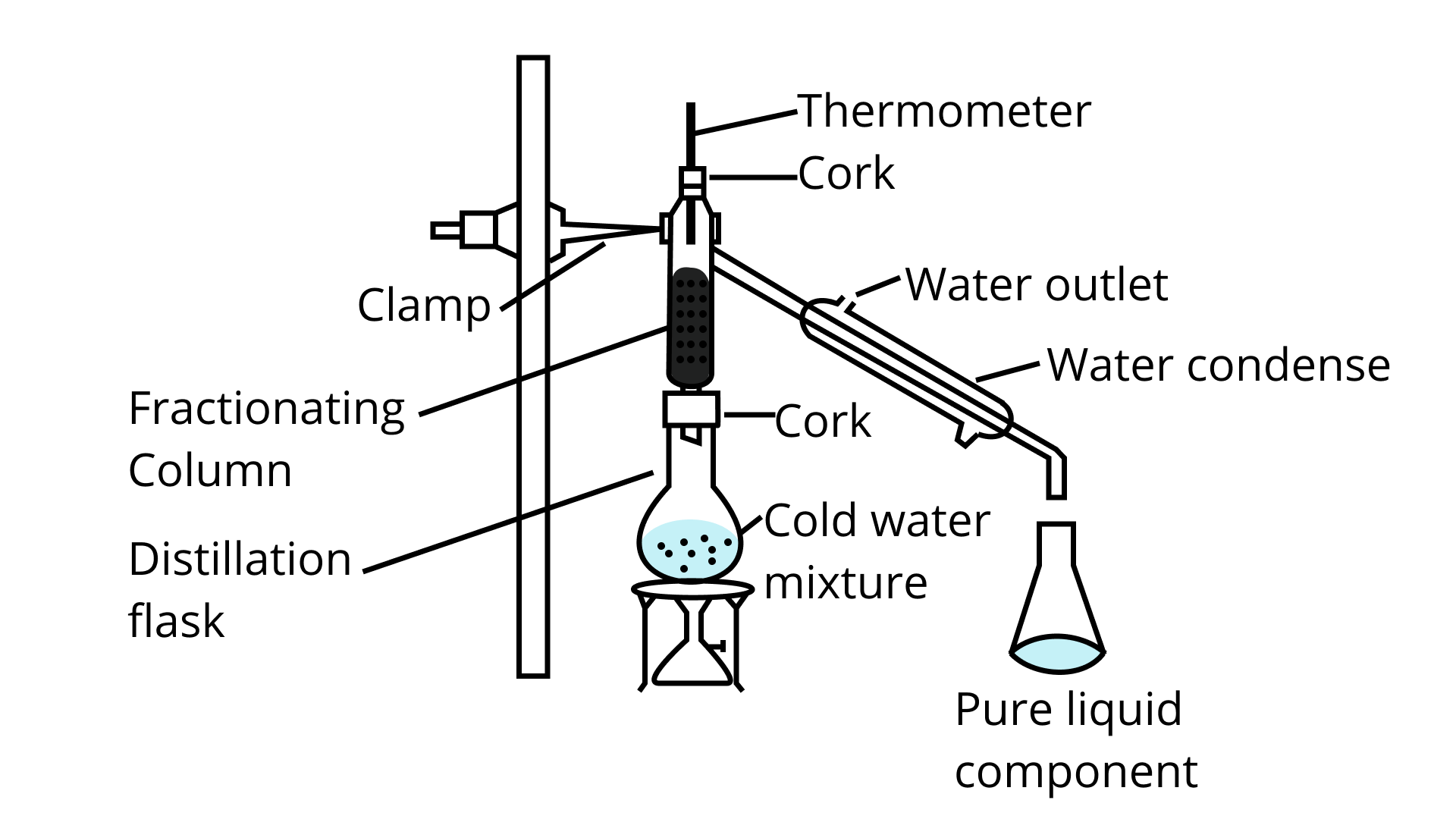
A fractionating column makes the process of the fractional distillation more efficient than the process of the simple distillation by providing increased possibilities for the condensation of the liquid. A fractionating column packed with glass beads or glass helices provides a large surface area for the vapours to collide and lose energy so that they can be condensed and can be distilled quickly. Increasing the length of the fractionating column would also increase the efficiency of the fractional distillation.
34. Answer the following:-
a. Under which category of mixtures will you classify alloys and why?
Ans: The alloys are homogeneous mixtures because they have a uniform composition throughout.
b. A solution is always a liquid. Comment.
Ans: No, a solution is not always a liquid. The solid solutions and the gaseous solutions are also possible. For example the brass is a solid solution and the air is a gaseous solution.
c. Can a solution be heterogeneous?
Ans: No, a solution cannot be heterogeneous. A solution is a homogenous mixture of two or more than two substances.
35. Iron filings and sulphur were mixed together and divided into two parts, ‘A’ and ‘B’. Part ‘A’ was heated strongly while Part ‘B’ was not heated. Dilute hydrochloric acid was added to both the Parts and evolution of gas was seen in both the cases. How will you identify the gases that evolved?
Ans: The following reaction will take place when part A (Mixture of Iron filings and sulphur) is heated:
Fe( s ) + S( s ) → Fe( s )
When the dilute hydrochloric acid is added to the mixture, the following reaction takes place and the hydrogen sulphide gas is evolved.
FeS( s ) + 2HCl(aq ) → FeCl2 (aq) + H2 S(g)
The hydrogen sulphide is a foul smelling gas and smells like rotten eggs.
When the dil. hydrochloric acid is added to the mixture of iron and sulphur (Part B), the following reaction takes place and the hydrogen gas is evolved. In this case, the sulphur does not participate in the reaction.
Fe(s) + S(s) + 2HCl(aq) → FeCl2(aq) + H2 (g) + S(s)
When a burning matchstick is brought near the evolved gas, the matchstick burns with a pop sound. This confirms the evolution of the hydrogen gas.
36. A child wanted to separate the mixture of dyes constituting a sample of ink. He marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in Fig. 2.3. The filter paper was removed when the water moved near the top of the filter paper.
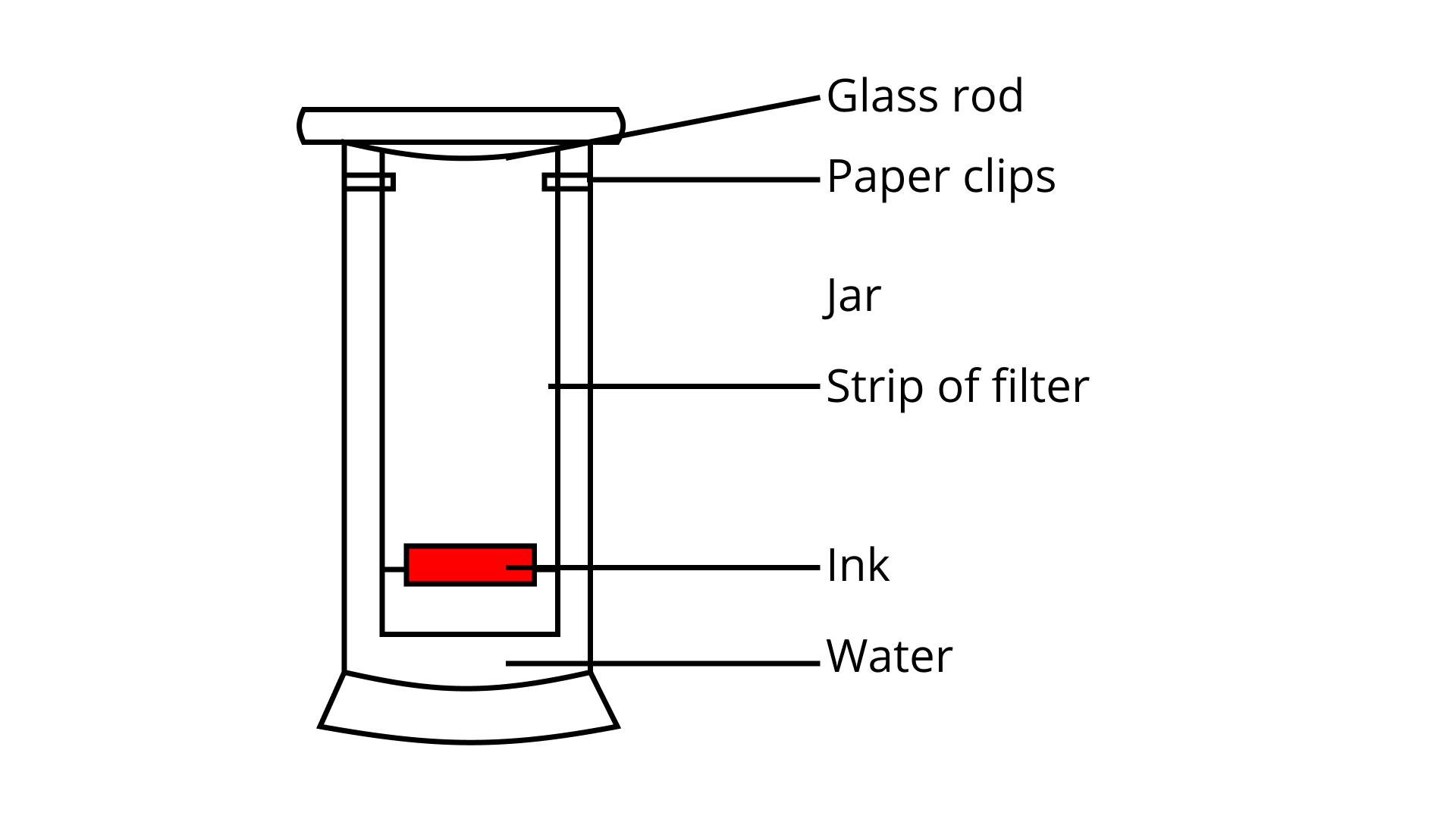
i. What would you expect to see, if the ink contains three different coloured components?
Ans: If the ink contains three different coloured components, three different bands will be seen on the filter paper.
ii. Name the technique used by the child.
Ans: The child used the technique of Paper Chromatography.
iii. Suggest one more application of this technique.
Ans: The paper Chromatography can be used to separate the pigments present in the chlorophyll.
37. A group of students took an old shoe box and covered it with a black paper from all sides. They fixed a source of light (a torch) at one end of the box by making a hole in and made another hole on the other side to view the light. They placed a milk sample contained in a beaker/tumbler in the box as shown in Fig.2.4. They were amazed to see that milk taken in the tumbler was illuminated. They tried the same activity by taking a salt solution but found that light simply passed through it?
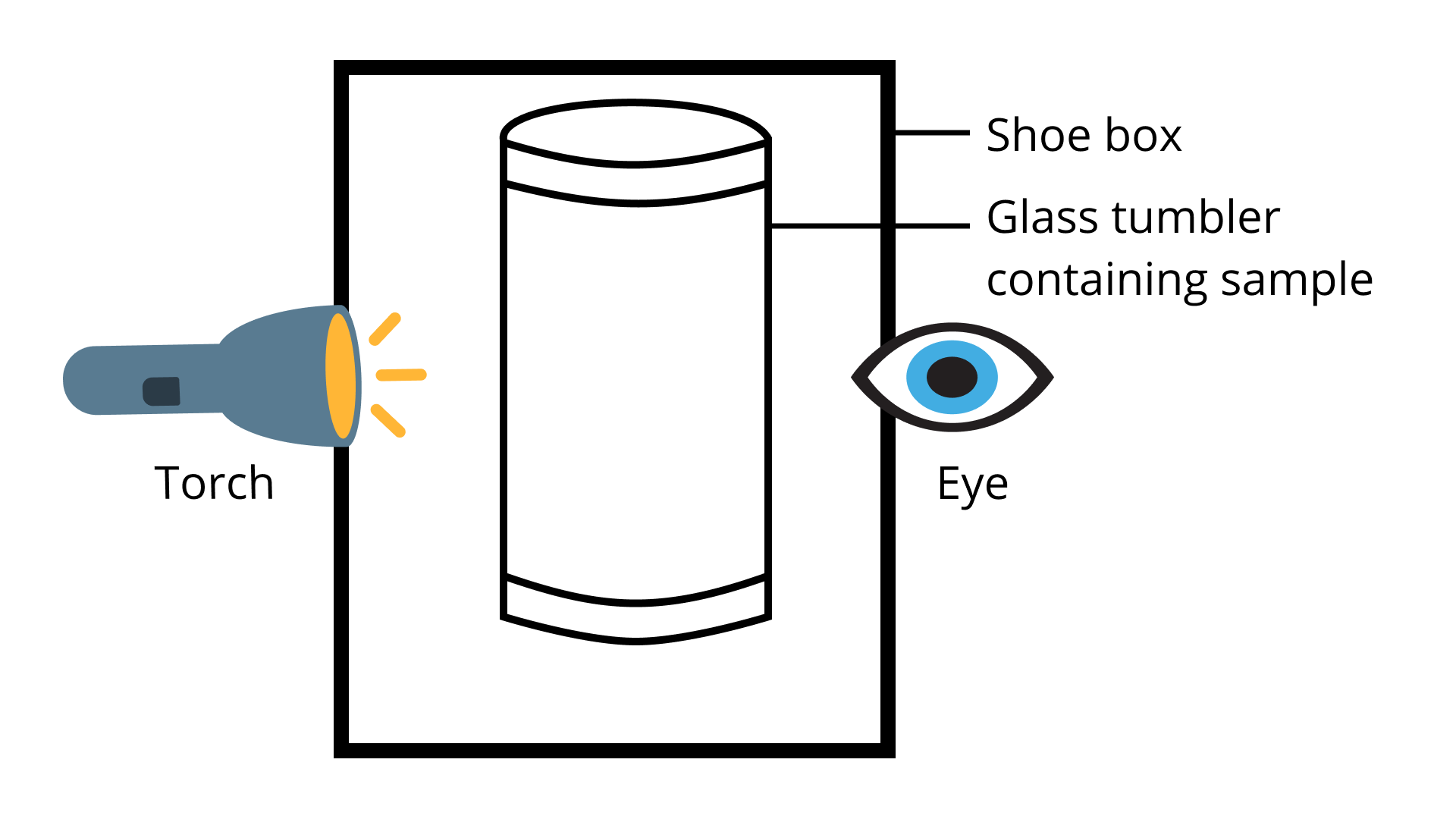
a. Explain why the milk sample was illuminated. Name the phenomenon involved.
Ans: The milk is a colloid. The particulate matter present inside milk scatters the light passing through the milk and shows Tyndall effect.
b. Same results were not observed with a salt solution. Explain.
Ans: The salt solution is a homogeneous solution. The small particles present in a salt solution do not scatter light and therefore a salt solution does not exhibit the Tyndall effect.
c. Can you suggest two more solutions which would show the same effect as shown by the milk solution?
Ans: The detergent solution and the sulphur solution will also show the Tyndall effect.
38. Classify each of the following, as a physical or a chemical change. Give reasons.
a. Drying a shirt in the sun.
Ans: The drying of a shirt in the sun: The physical change. There is no chemical reaction that happens during the drying of a shirt under the sun.
b. Rising of hot air over a radiator.
Ans: The rising of hot air over a radiator: The physical change. The water in a radiator converts to vapours. The hot air becomes lighter and rises.
c. Burning of kerosene in a lantern.
Ans: The burning of kerosene in a lantern: The chemical change. The kerosene burns and combines with the atmospheric oxygen to form new chemical products.
d. Change the colour of black tea by adding lemon juice to it.
Ans: Change in the colour of black tea by adding lemon juice to it: The chemical change.
The lemon is a source of citric acid, ascorbic acid and malic acid. The black tea contains theaflavin antioxidants.
e. Churning of milk cream to get butter.
Ans: The churning of milk cream to get butter: The physical change. There is no chemical reaction that happens during the centrifugation of milk cream.
39. During an experiment the students were asked to prepare a 10% (Mass/Mass) solution of sugar in water. Ramesh dissolved 10 g of sugar in 100 g of water while Sarika prepared it by dissolving 10 g of sugar in water to make 100 g of the solution.
a. Are the two solutions of the same concentration?
Ans: No, the two solutions will not have the same concentration.
Mass % = \[\mathrm{\frac{mass\: of\: solute}{\textrm{[mass of solute + mass of solvent]}}\times 100}\]
b. Compare the mass % of the two solutions.
Ans: Mass % of solution made by Rames
= \[\mathrm{\frac{10}{110}\times 100}\]
= 9.09%
Mass % of solution made by Sarika
= \[\mathrm{\frac{mass\: of\: solute}{\textrm{[mass of solute + mass of solvent]}}\times 100}\]
\[\mathrm{\frac{10}{110}\times 100}\]
= 9.09%
Therefore the solution prepared by Sarika has a higher mass % than that prepared by Ramesh.
40. You are provided with a mixture containing sand, iron filings, ammonium chloride and sodium chloride. Describe the procedure you would use to separate these constituents from the mixture?
Ans: The components of the given mixture can be separated by the following methods:-
i. By using a magnet: By moving a magn.et over the mixture will result in the iron fillings getting stuck to the magnet. Hence the iron will be separated from the mixture.
ii. By the sublimation: The remaining mixture is heated in a china dish. The ammonium chloride is a sublimating substance and therefore it will evaporate without passing through the liquid phase. The crust of ammonium chloride can be collected by placing an inverted funnel on top of the china dish.
iii. By the sedimentation, decantation and filtration: The remaining mixture is dissolved in the water and allowed to settle for some time. The sand, being insoluble in water, settles at the bottom. The liquid is decanted in the other beaker. The liquid is then filtered to remove any traces of sand.
iv. By evaporation: The liquid is now a solution of salt in water. This is heated in a beaker so that the water evaporates. Once all the water evaporates, salt remains in the beaker.
41. Arun has prepared 0.01% (by mass) solution of sodium chloride in water. Which of the following correctly represent the composition of the solution?
1.00 g of NaCl + 100 g of water
0.11 g of NaCl + 100 g of water
0.01 g of NaCl + 99.99 g of water
0.10 g of NaCl + 99.90 g of water
Ans: (c) 0.01g of NaCl + 99.99 g of water is the correct composition of the solution.
Mass % in (C) = \[\mathrm{\frac{mass\: of\: solute}{\textrm{[mass of solute + mass of solvent]}}\times 100}\]%
= \[\mathrm{\left ( \frac{.01}{.01+99.99} \right )\times 100}\]
= 0.01%
42. Calculate the mass of sodium sulphate required to prepare its 20% (mass percent) solution in 100 g of water?
Ans: Let the mass of the sodium sulphate required to prepare the solution be "x" grams.
The mass of the solvent (water) is given as 100 g .
The mass of the solution would be (x +100)g
x gof solute (sodium sulphate) is dissolved in (x+100) g of solution
20% = x / [x +100]×100 Or, 20x + 2000 =100x Or, 80x = 2000
Or, x = 2000 / 80
Or, x = 25g
Hence 25g of sodium sulphate will be required to prepare its 20% solution in 100 g
of water.
Introduction to Chapter 2
Chapter 2 of class 9 science begins with an introduction of what matter is. Pure and impure substances have then been stressed upon in detail. Then, students learn about what metals and nonmetals are. A comparative table of their properties has then been explained. Some elements have properties of both metals as well as non-metals and are known as metalloids. That too has been explained. We then learn about the types of the mixture and the distinction between a compound and a mixture. Illustrations have been used for a clearer understanding. Solutions, suspensions, and colloids have then been explained. The chapter ends after the Separation of Mixtures has been dealt with.
FAQs on NCERT Exemplar for Class 9 Science - Is Matter Around Us Pure - Free PDF Download
1. What are the kinds of questions that have been asked in NCERT Exemplar for Class 9 science- Is matter around us pure?
There are multiple-choice questions, short answer questions, long answer questions in NCERT Exemplar for Class 9 science- Is matter around us pure.
These questions have been designed by the various scientific experts and science teachers as per the NCERT guidelines. They help students prepare for all sorts of examinations including competitive examinations. The book is available on Vedantu and is completely free of cost. All the study material on Vedantu is free of cost so that the students do not hesitate before getting their hands on quality academic material.
2. How are metals and non-metals different from each other?
Metals and nonmetals are both different from each other as they have different properties. Some examples of metals are iron, copper, and aluminium and some non-metals would be wood, rubber, glass.. The distinction between them and their full-fledged explanations are provided on Vedantu.com. The site has the complete study material that’s needed by students of Class 9 for science. The explanations are well and to the point. This facilitates smoother learning in students who wish to score higher grades.
3. How can one understand the concepts of NCERT science in Class 9 apart from the main textbooks?
One can check out NCERT Exemplar for Class 9 science on Vedantu.com as the revision notes and the solved questions are very effective when it comes to appearing for examinations. Students always need a revision book of some sort to go through right before their tests for a comprehensive understanding of the chapters that are there. Unless a particular concept is clear, it will become very tough for students to answer challenging questions that are posed to them. The books present on Vedantu have just the right amount of information that’s needed and have been made as per the NCERT guidelines.
4. Is common salt an organic compound?
Organic compounds are derived from living organism parts whereas inorganic compounds are derived from nonliving materials such as rocks and minerals. Common salt is an inorganic compound as it comes from sodium chloride mineral halites. The explanations for these have been summed up in NCERT Exemplar for Class 9 Science Chapter 2 - Is Matter Around Us Pure. The concepts have been simplified down to the students’ level of comprehension and are pretty interesting to go through. You can find the book online on Vedantu.
5. How do I make revision notes for NCERT Class 9 Science Chapter 2?
You can make revision notes by logging into Vedantu.com where you will find NCERT Exemplar for Class 9 Science Chapter 2 - Is Matter Around Us Pure. The chapter has revision notes as well as solved questions in all formats for you to learn. The language used is simple and scientific and the solved questions will prepare you for all those tests that you might have to take. You must go through each and every solved question to understand the chapter completely and make relevant notes for revision.





































