Free Download of Solutions In PDF Available From Vedantu
Free download of step-by-step solutions for class 10 Science (Chemistry) Chapter 12 - Organic Chemistry of ICSE Board (Concise - Selina Publishers). All exercise questions are solved & explained by an expert teacher and as per ICSE board guidelines.
Solutions for ICSE Class 10 Chemistry Chapter 12 “Organic Chemistry” is available for free download at Vedantu.
Council for the Indian School Certificate Examination (CISCE) is a board of school education in India and it is private, the Indian certificate of secondary education and the Indian School certificate are the two exams that CISCE conducts.
Concise Selina Publication publishes textbooks that are prepared after taking into consideration all the guidelines provided by the Council for the Indian School Certificate Examination (CISCE).
Chapter 12 of the Class 10 Chemistry, ICSE Board (Concise – Selina Publishers).
Intext Questions
1. (a) What are organic compounds?
Ans: Any chemical substance with carbon-hydrogen bonds is considered an organic compound. Organic chemistry is the science that studies the characteristics, reactions, and syntheses of organic substances.
(b) What is vital force theory? Why was it discarded?
Ans: The Vital Force Theory was proposed by Berzelius in 1809, and it states that organic chemicals can only be created in live cells and that preparing them in laboratories is impossible. Friedrich Wohler demonstrated that an organic chemical (urea) could be produced in the laboratory, thus it was discarded.
2. (a) Name a few sources of organic compounds.
Ans: Plants, animals, coal, petroleum, and wood are all sources of organic molecules.
(b) Give the various applications of organic chemistry.
Ans: Organic chemistry is utilised in the manufacturing of soaps, shampoos, powders, and perfumes, among other things. Organic chemicals are also found in other fuels such as natural gas and petroleum. Organic compounds are also used in the fabrics that we employ to produce diverse garments.
3. Organic chemistry plays a key role in all walks of life. Discuss
Ans: Organic compounds can be found in almost any environment.
They can be found in:
1. It's used to make soaps, shampoos, powders, and perfumes, among other things.
2. It is found in foods such as carbs, proteins, lipids, and vitamins.
3. Drugs, explosives, dyes, and insecticides are all prohibited.
4. Petroleum and natural gas are both organic substances.
As a result, organic substances are important in many aspects of existence.
4. Carbon shows some unique properties, name them.
Ans: Carbon has the following distinctive properties:
1) Tetravalency of carbon
2) Catenation.
3) Isomerism.
5. Explain the following:
(a) Tetravalency
Ans: To achieve octet, carbon cannot lose or receive electrons. As a result, it has four electrons in common with other atoms. Tetravalency of carbon refers to the property of carbon that allows it to make four covalent connections.
(b) Catenation
Ans: The bonding of atoms of the same element into a chain is known as catenation. An open-chain compound is one in which the ends of a chain or ring are not linked to each other, whereas a closed-chain compound is one in which the ends are bonded in a ring (a cyclic compound).
6. Write any four properties of organic compounds that distinguish them from inorganic compounds.
Ans: Organic substances have four characteristics that set them apart from inorganic compounds:
Carbon is present.
They are soluble in organic solvents.
Covalent bonds are formed.
The melting and boiling points are both low.
7. Why are organic compounds studied as a separate branch of chemistry?
Ans: Because of the particular nature of the carbon atom, a huge number of compounds can be formed. As a result, a new field of chemistry is required.
8. What are hydrocarbons? Compare saturated and unsaturated hydrocarbons?
Ans: Saturated hydrocarbons have only single carbon-carbon bonds. Unsaturated hydrocarbons have double or triple carbon-carbon bonds (more hydrogens can be added).
9. Give reason for the existence of the large number of organic compounds.
Ans: Catenation, or the formation of covalent bonds with other carbon atoms, is a simple process that allows carbon to create longer chains and greater mass structures. This is the cause for the large amount of carbon-based organic molecules found in nature.
10. Give at least one example in each case to show the structure of isomers of:
(a) Single bond compound
Ans: n-Pentane
(b) Double bond compound
Ans: Ethene
(c) Triple bond compound
Ans: Ethyne
11. Name a compound of each type and draw the figure,
(a) Saturated hydrocarbons
Ans: n-Pentane
(b) Unsaturated hydrocarbons
Ans: Ethyne
12. Define substitution and addition reactions. Give an example for each.
Ans: Addition reactions are the reaction, in which reagent is added by breaking the unsaturated bonds (double or triple bond) e.g: By adding hydrogen in the presence of a catalyst, unsaturated hydrocarbons can be converted to saturated hydrocarbons. Substitution reactions are reactions in which a reagent substitutes one atom or a group of atoms from the reactants. e. g. In the presence of sunlight, CH4+Cl2 will produce CH3Cl+HCl.
Intext Questions
1. Define a functional group and give the structural formula of the following functional groups:
Ans: Functional group is a group of atoms that are responsible for the characteristic reactions of a particular compound.
(a) Ketone
Ans:
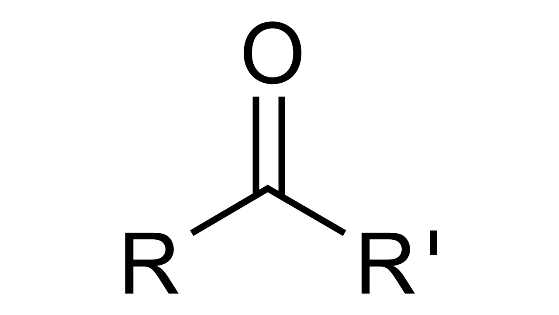
(b) Alcohols
Ans: R-OH
(c) Aldehydes
Ans: R-CH=O
2. Write the name and formula of fourth member of the following homologous series:
(a) Alkyne
Ans: Butyne C4H6
(b) Alcohol
Ans: Butanol C4H9OH
3. Which part of an organic compound determines
(i) Physical properties
Ans: Alkyl part
(ii) Chemical properties
Ans: Functional group
4. Name the alkyl radical and the functional group of the following organic compounds:
(a) CH3OH
Ans: Alkyl radical- Methyl (CH3.)
Functional group- alcohol (OH)
(b) C2H5OH
Ans: Alkyl radical- Ethyl (C2H5.)
Functional group- alcohol (OH)
(c) C3H76CHO
Ans: Alkyl radical- Propyl
Functional group- Aldehyde
(d) C4H9COOH
Ans: Alkyl radical- Butyl
Functional group- carboxyl
(e) CH3COOH
Ans: Alkyl radical- CH3.
Functional group- Carboxyl
(f) HCHO
Ans: Alkyl radical- H.
Functional group- Aldehyde
5. (a) What is an alkyl group?
Ans: A group of atoms formed by the loss of a hydrogen atom from an alkane is called alkyl group.
(b) Give the names of any three alkyl radicals. How are they formed?
Ans: Three alkyl radicals are: Methyl, ethyl, Propyl. These are formed by losing one hydrogen atom.
CH4→CH3. + H.
C2H6→C2H5. + H.
C3H8→C3H7. + H.
6. Give the names and the structural formula of the first three members of the homologous series of alkanes.
Ans: Methane (CH4), ethane(C2H6), and propane (C3H8) are the first three members of the homologous series of alkanes.
7. (a) What is a homologous series?
Ans: A homologous series is a collection of organic compounds with identical structures and chemical characteristics that differ only by the presence of a CH2 group.
(b) What is the difference in the molecular formula of any two adjacent homologues:
(i) In terms of molecular mass.
Ans: The molecular formula difference between any two neighbouring homologues is 14 a.m.u.
(ii) In terms of number and kind of atoms per molecule?
Ans: It is three atoms apart. It differs by one carbon atom and two hydrogen atoms. So, two kinds of atoms.
Exercise- 12 A
1. Write the IUPAC name of the following:
(a)
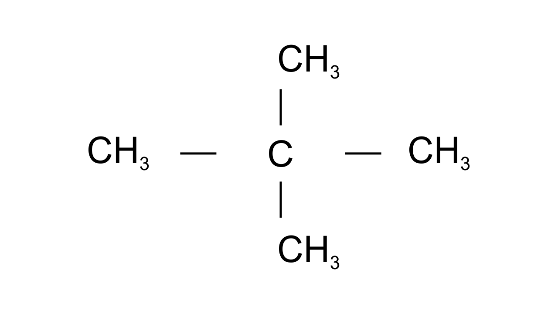
Ans: 2,2-Dimethylpropane
(b)
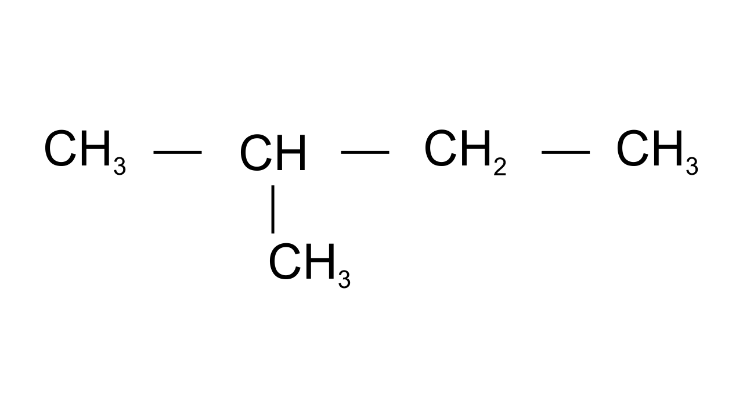
Ans: 2-Methylbutane
(c)
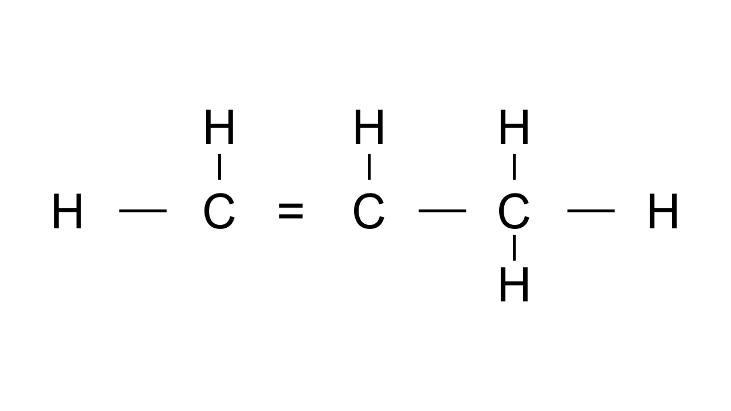
Ans: Prop1-ene
(d)
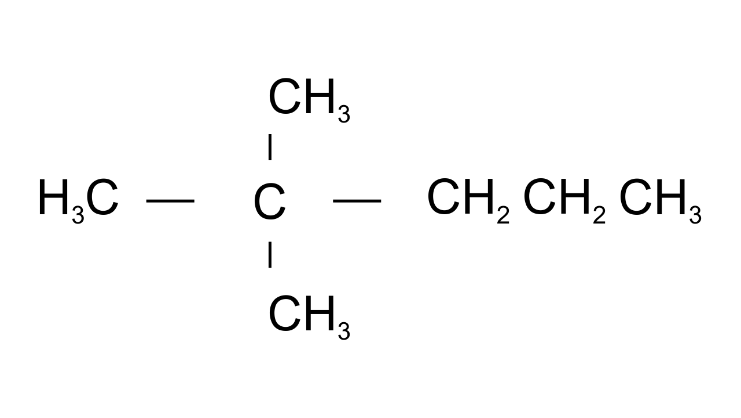
Ans: 2,2-Dimethylpentane
(e)
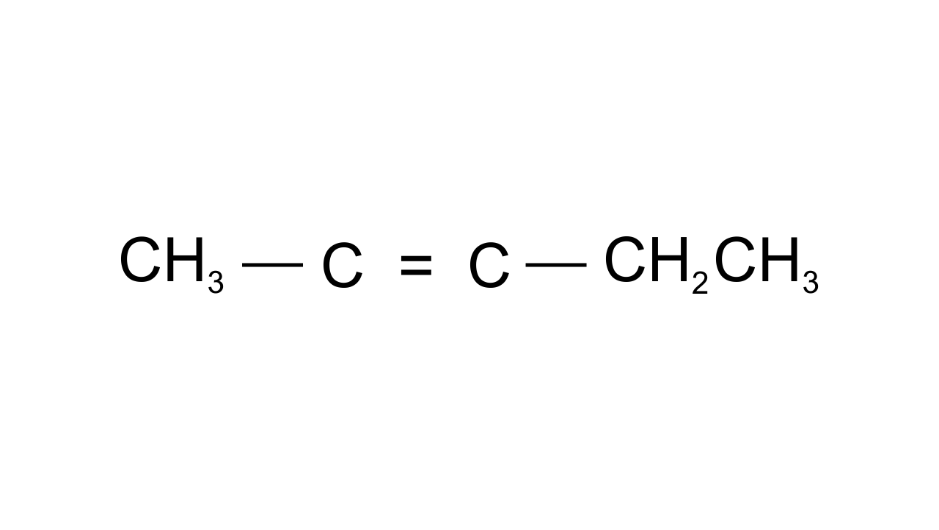
Ans: Pent2-ene
(f)
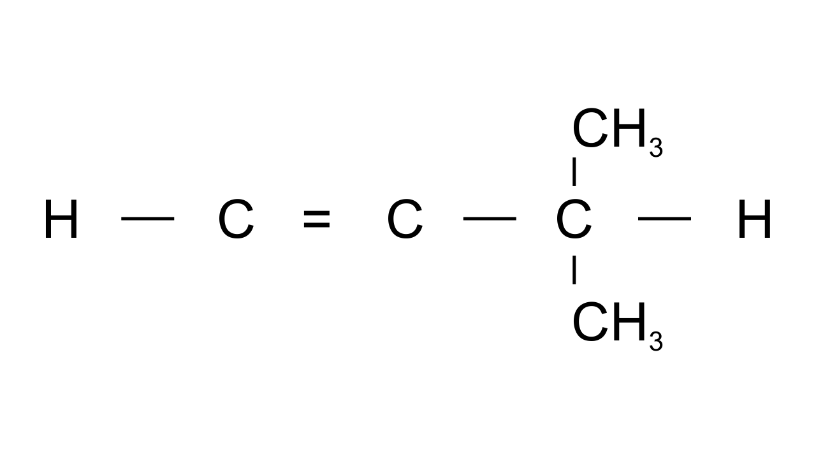
Ans: 3-Methylbut1-ene
(g)
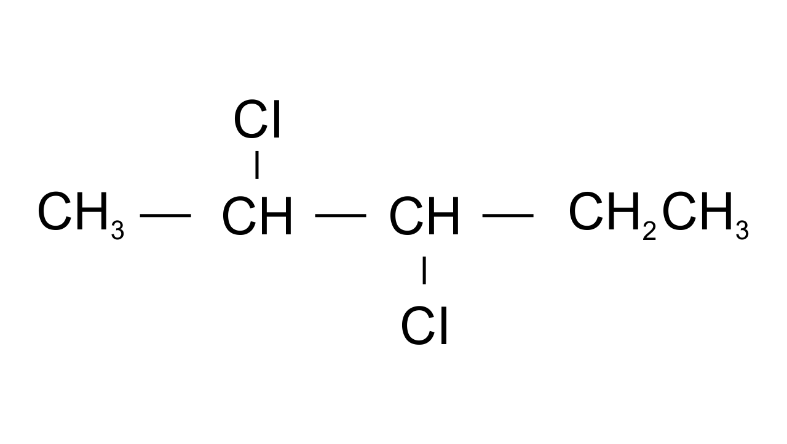
Ans: 2,3-Dichloropentane
(h)
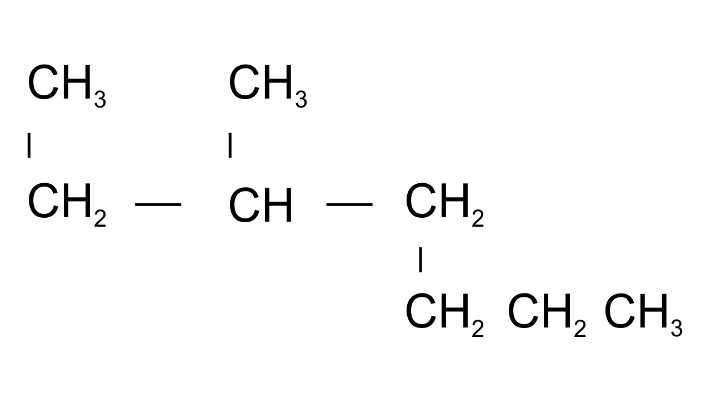
Ans: 2-Methylheptane
(i)
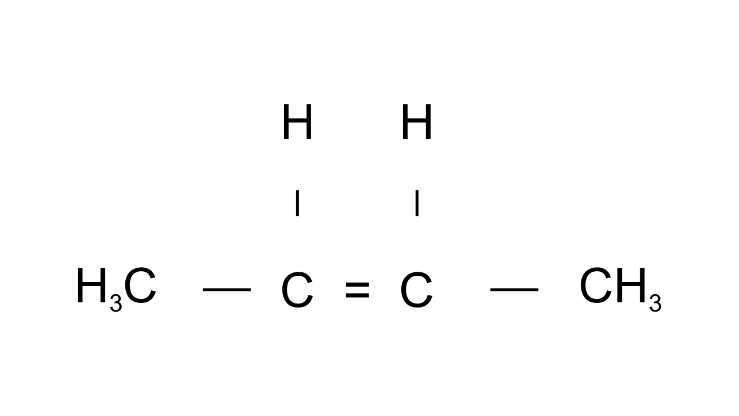
Ans: But2-ene
(j) \[C{{H}_{3}}-C=C-C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
Ans: Hept2-ene
(k)
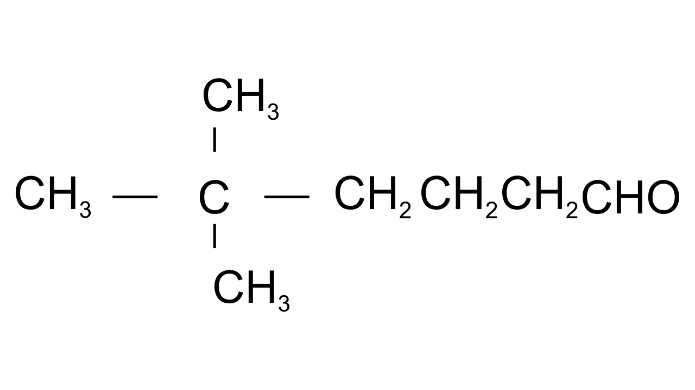
Ans: 5,5-Dimethylhexaneal
(l)
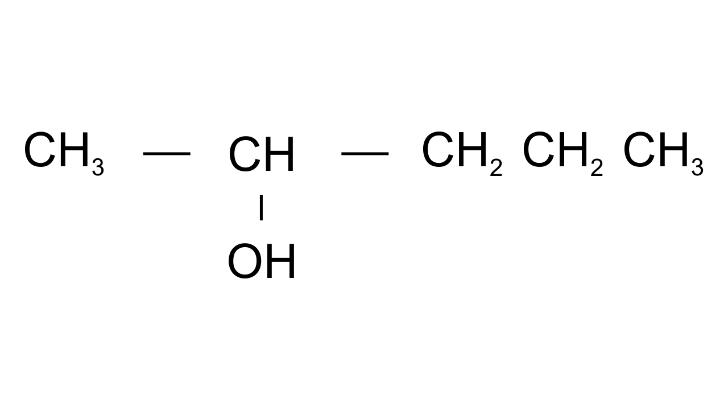
Ans: Pentan2-ol
(m)
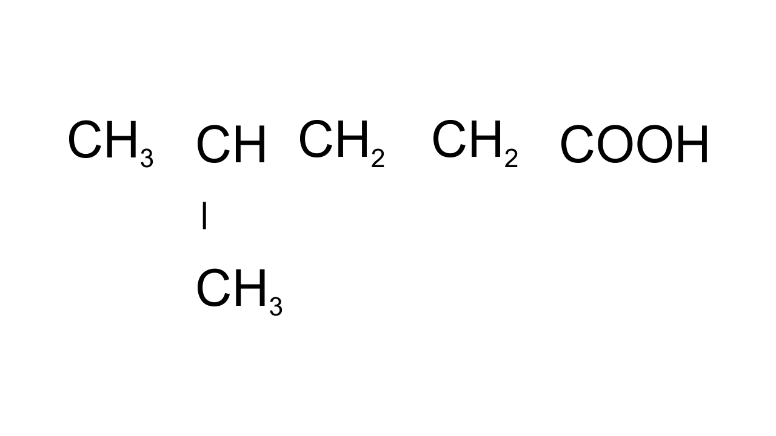
Ans: 4-Methylpentanoic acid
(n)
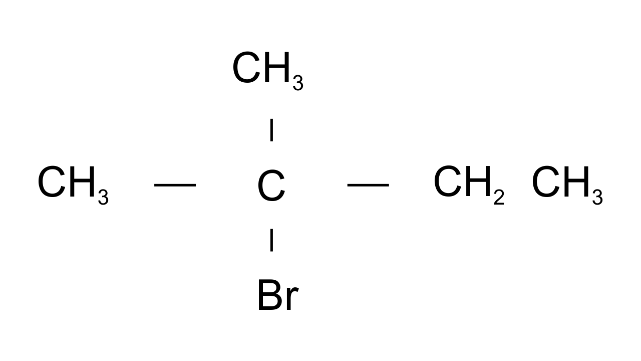
Ans: 2-Bromo2-methylbutane
(o)
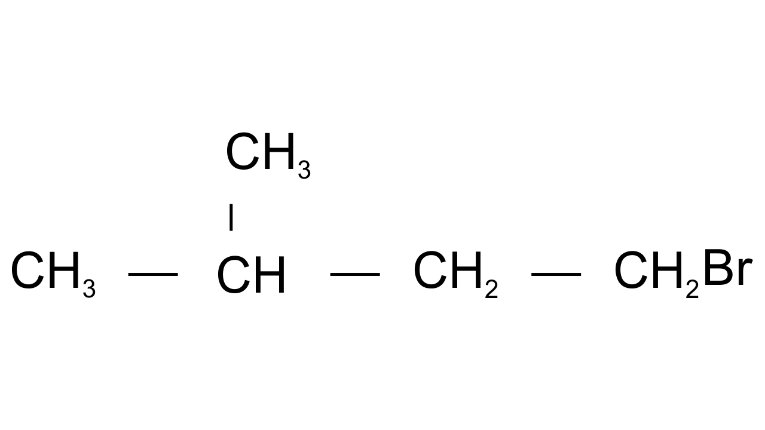
Ans: 1-Bromo3-methylbutane
(p)
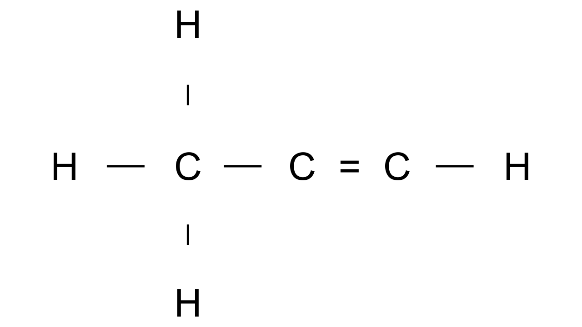
Ans: Prop1-ene
(q)
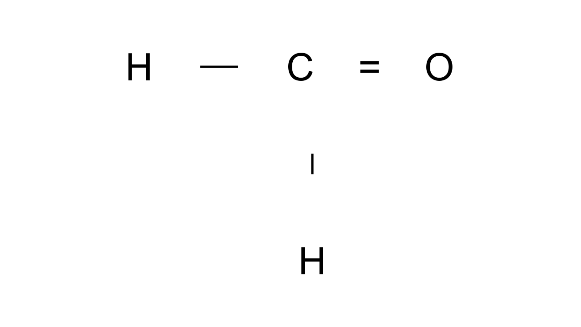
Ans: Methane-al
(r)
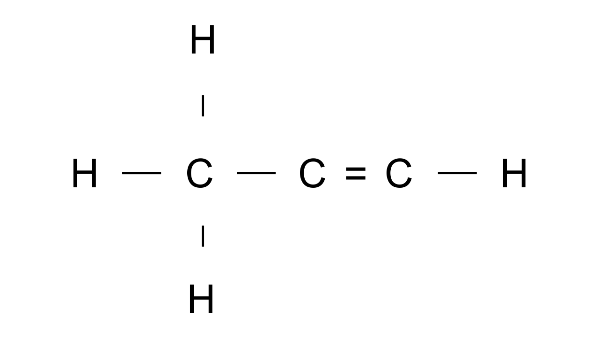
Ans: Prop1-ene
(s)
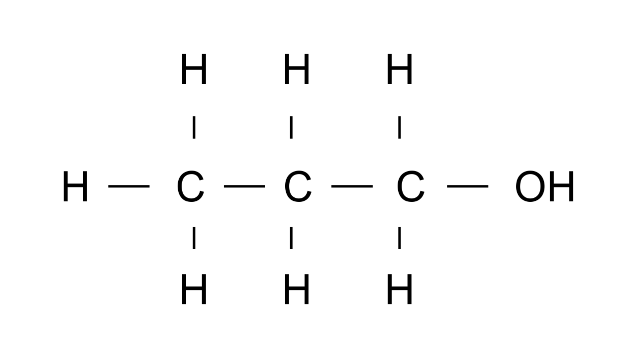
Ans: Propan1-ol
(t)
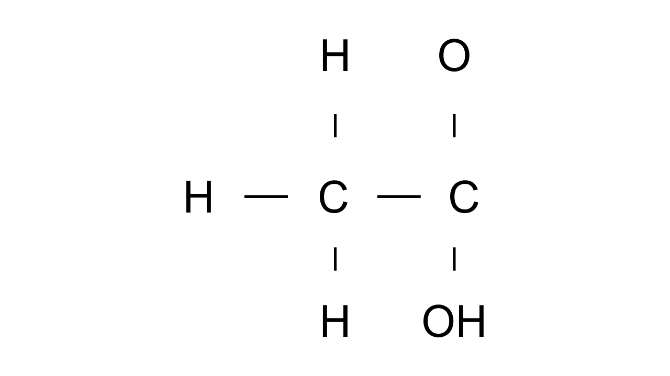
Ans: Ethanoic acid
(u)
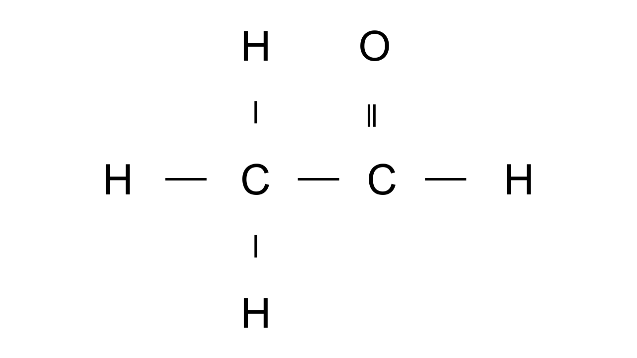
Ans: Ethanal
(v)
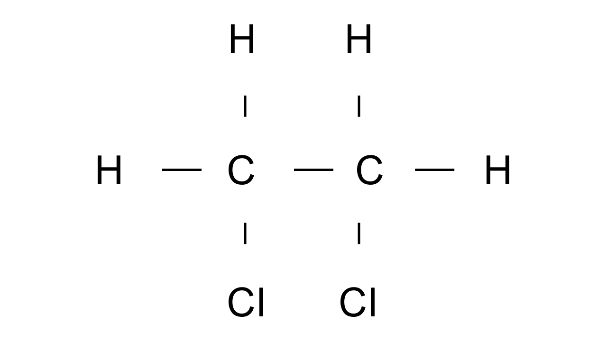
Ans: 1,2-Dichloroethane
2. Write the structures of the following compounds:
(a) Prop-1-ene
Ans:
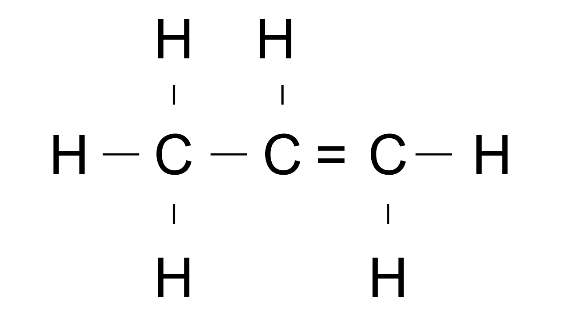
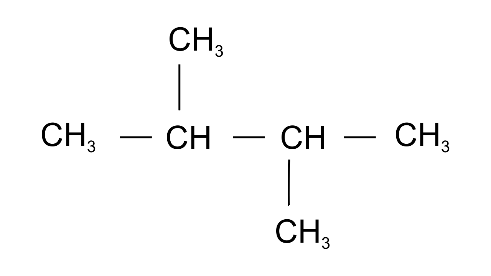
(b) 2,3-dimethylbutane
Ans:
(c) 2-methylpropane
Ans:
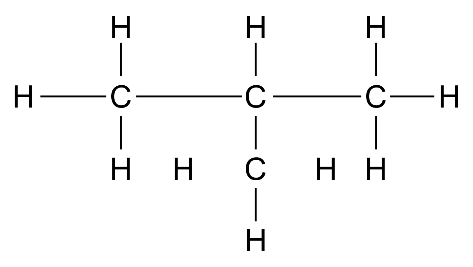
(d) 3-hexene
Ans: \[C{{H}_{3}}C{{H}_{2}}CH=CHC{{H}_{2}}C{{H}_{3}}\]
(e) Prop-1-yne
Ans:
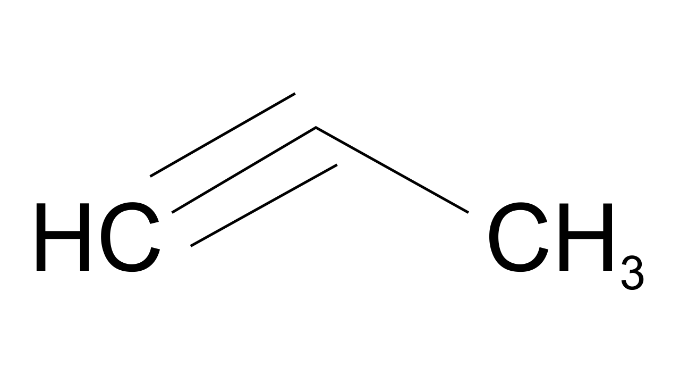
(f) 2-methylprop-1-ene
Ans:
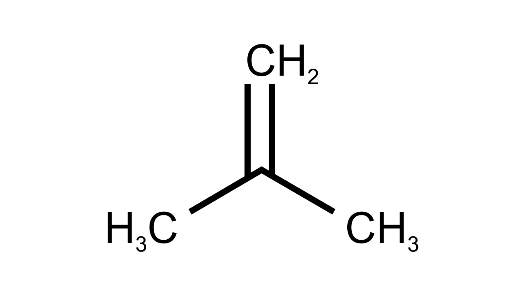
(g) Alcohol with molecular formula C4H10O
Ans:
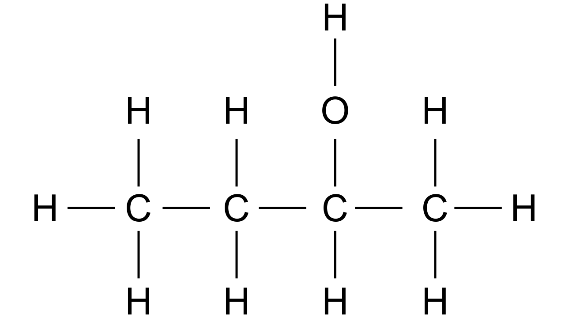
3. Choose the correct answer:
(a) C5H11 is an
(i) alkane
(ii) alkene
(iii) alkyne
(iv) alkyl group
Ans: Alkyl group
(b) A hydrocarbon of the general formula CnH2n is
(i) C15H30
(ii) C12H26
(iii) C8H20
(iv) C6H14
Ans: C15H30
(c) The total number of different carbon chains that four carbon atoms from in alkane is
(i) 5
(ii) 4
(iii) 3
(iv) 2
Ans: 2
(d) CH3-CH2-OH and CH3-O-CH2 are
(i) Position isomers
(ii) Chain isomers
(iii) homologous
(iv) functional group isomers
Ans: functional group isomers
(e) The IUPAC name of the compound is
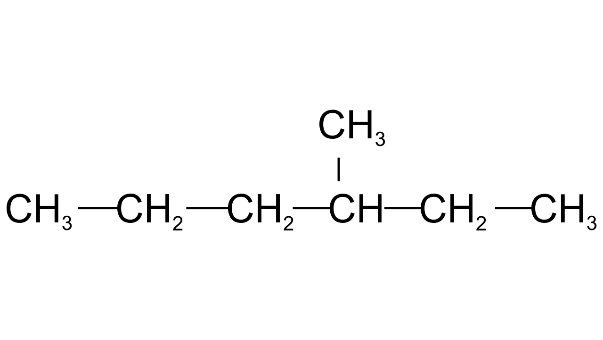
(i) 3-trimethylhexane
(ii) 3-methyl hexane
(iii) 4-methyl hexane
Ans: 3-trimethylhexane
4. Fill in the blanks.
(a) Propane and ethane are……..(homologous, isomers)
Ans: Homologous
(b) A saturated hydrocarbon does not participate in a/an ……….reaction (substitution, addition)
Ans: Substitution
(c) Succeeding members of homologous series differ by………… (CH2, CH, CH3)
Ans: CH2
(d) As the molecular masses of hydrocarbons increase, their boiling points……… and melting points……….. (increase, decrease)
Ans: increase
(e) C25H52 and C50H102 belong to ……… homologous series (the same, different)
Ans: Same
(f) CO is an ………… compound. (organic, inorganic)
Ans: Inorganic compound
(g) The chemical properties of an organic compound are largely decided by the……… and the physical properties of an organic compound are largely decided by the …….. (functional group, number of carbon atom)
Ans: Functional group, number of carbon chain
(h) CHO is the functional group of an ……….. (alcohol, aldehyde)
Ans: Aldehyde
(i) The root in the IUPAC name of an organic compound depends upon the number of carbon atoms in ………. (any chain, principal chain)
Ans: Principal chain
(j) But 1-ene and but-2-ene are examples of ……… isomerism (chain, position, functional)
Ans: Position
5. Define or explain chain isomerism and position isomerism with examples in each case.
Ans: Chain isomerism arises due to the difference in arrangement of C atoms in the chain. For example, there are two isomers of butane, C4H10. In one of them, the carbon atoms lie in a straight chain whereas in the other the chain is branched.
Position isomerism is due to the difference in position of functional groups. For example, there are two structural isomers with the molecular formula C3H7Br. In one of them, the bromine atom is on the end of the chain, whereas in the other it is attached in the middle.
6. (a) Define the term isomerism. State two main causes of isomerism.
Ans: Compounds having the same molecular formula but different structural formula are known as isomers and the phenomenon as isomerism. Two main causes of isomerism.
Difference is the mode of linking of atoms.
Difference in the arrangement of atoms or groups in space.
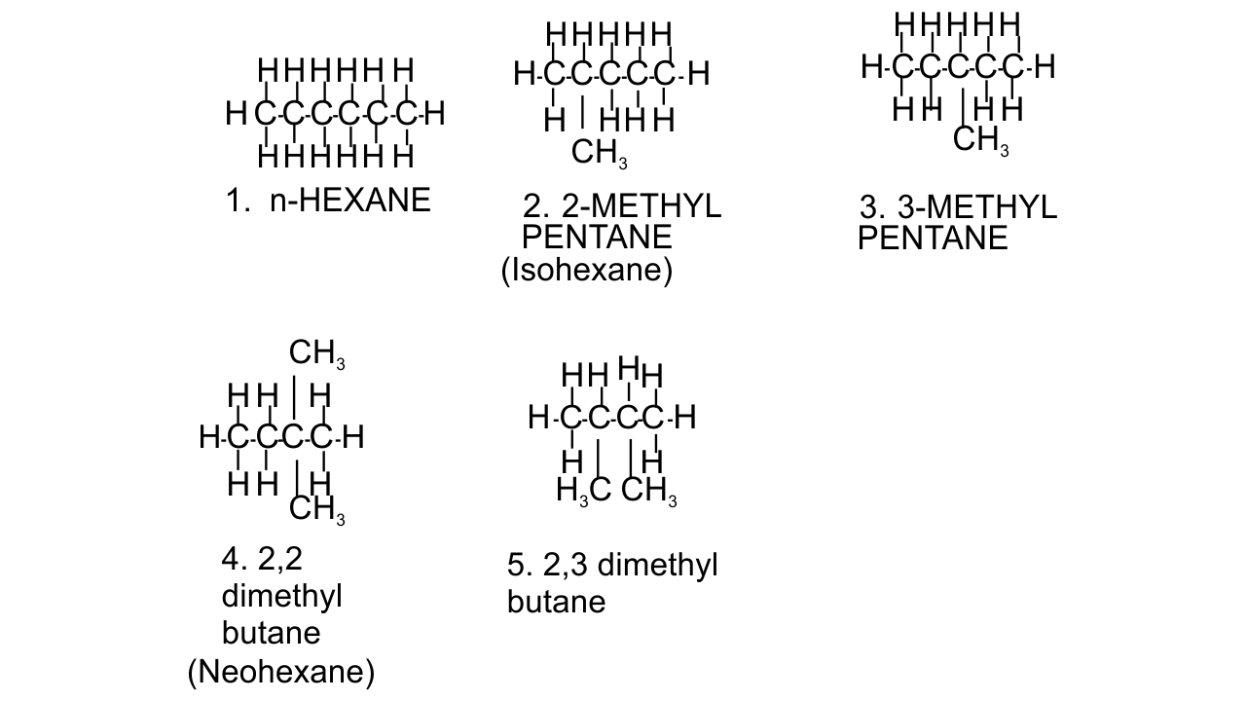
(b) Draw the chain isomers of hexane (C6H14).
Ans:
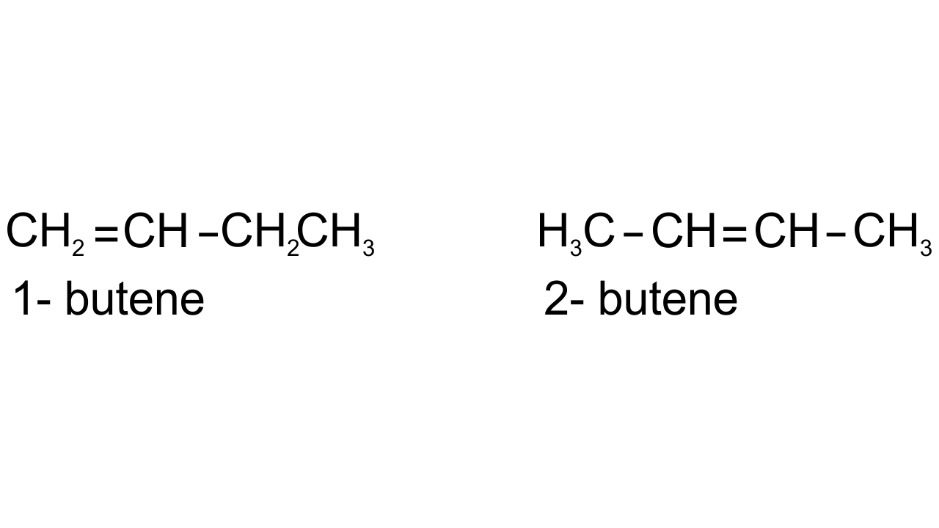
(c) Draw position isomers of butene (C4H8)
Ans:
7. Draw structural formula for each of the following compounds:
(a) isomer of n-butane
Ans: CH3-CH2-CH2-CH3
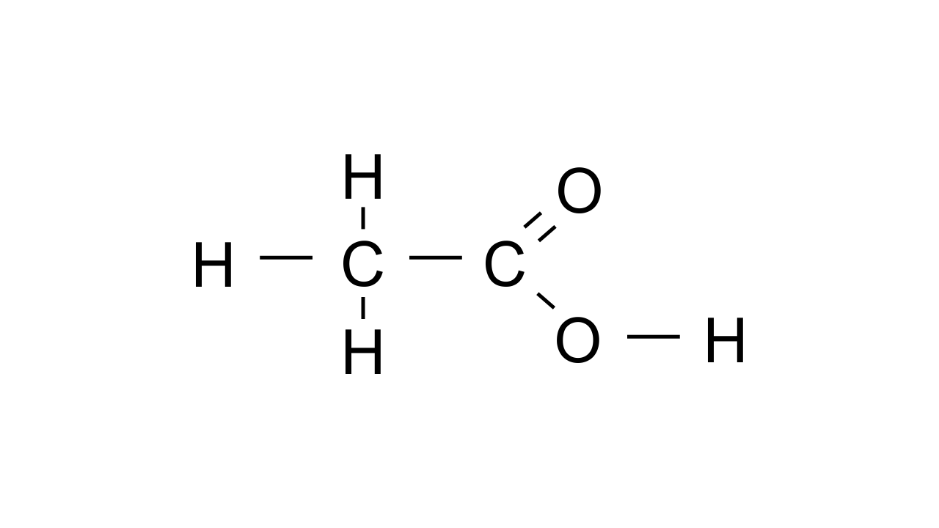
(b) Vinegar
Ans:
(c) 2-Propanol
Ans: CH3-CH (OH)-CH3
(d) Ethanal
Ans: CH3-CHO
(e) Acetone
Ans: CH3-C (O)-CH3
(f) Diethyl ether
Ans: CH3-CH2-O-CH2-CH3
What is used to describe these compounds together?
Ans: These compounds together can be called organic compounds.
8. (a) What is the special feature of the structure of:
(i) Ethene
Ans: Ethene possesses a double bond. The two carbon atoms in ethene relate to each other with a double bond.
(ii) Ethyne
Ans: Ethene possesses a triple bond. The two carbon atoms in ethene are connected with each other with a triple bond.
(b) What type of reaction is common to both of the above compounds? Why methane does not undergo this type of reaction.
Ans: The above compounds undergo addition reactions. Methane does not undergo this type of reaction due to the absence of unsaturated bonds (double and triple).
(C) What is the IUPAC name of dimethyl ether.
Ans: Methoxymethane
9. Which type of reaction will
(i) Ethane
(ii) Ethene
Undergoes?
Ans: Ethene undergoes addition reactions with reagents whereas ethane undergoes substitution reactions.
10. Choosing only words from the following list. Write down appropriate words to fill in the blanks from (a) to (e) given below: Addition, carbohydrates, CnH2n-2, CnH2n, CnH2n+2, electrochemical homologous, hydrocarbon, saturated, substitution, unsaturated.
The alkane from an (a) …….. Series with the general formula (b) ……… The alkanes are (c)..........(d) ………….which generally undergoes (e)..........reactions.
Ans: (a) Electrochemical homologous
(b) CnH2n+2
(c) saturated
(d) hydrocarbon
(e) Substitution
11. Draw the structural formula of a compound with two carbon atoms in each of the following cases.
(a) An alkane with a carbon to carbon single bond.
Ans: CH3-CH3
(b) An alcohol containing two carbon atoms.
Ans: CH3-CH2OH
(c) An unsaturated hydrocarbon with a carbon to carbon triple bond.
Ans: \[H-C\equiv C-H\]
12. Ethane, ethene, ethanoic acid, ethyne, ethanol from the above, name
(a) The compound with -OH as the part of its structure.
Ans: Ethanol
(b) The compound with -COOH as the part of its structure.
Ans: Ethanoic acid
(C) Homologue of homologous series with general formula CnH2n.
Ans: Ethene
13. Give the correct IUPAC name and the functional group for each of the compounds whose structural formulae are given below:
(a)
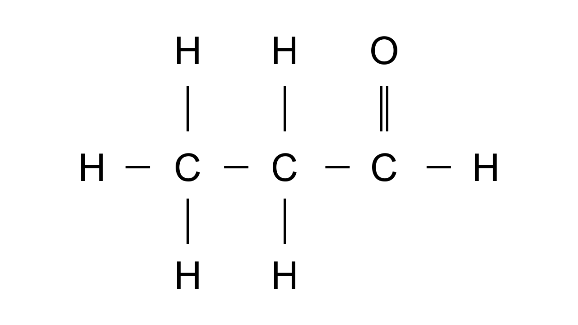
Ans: IUPAc name: Propanoic acid
Functional group: -COOH
(b)
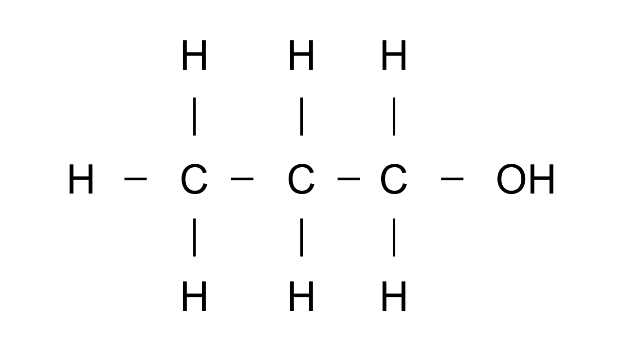
Ans: IUPAc name: Propanol
Functional group: -OH
14. Copy and complete the following table which relates to three homologous series of hydrocarbons:
General Formula | CnH2n | CnH2n-2 | CnH2n+2 |
IUPAc name of the homologous series | |||
Characteristic bond type | Single bonds | ||
IUPAC name of the first member of the series | |||
Type of reaction with chlorine | Addition |
Ans:
General Formula | CnH2n | CnH2n-2 | CnH2n+2 |
IUPAc name of the homologous series | Alkenes | Alkyne | Alkane |
Characteristic bond type | Double bond | Triple bond | Single bonds |
IUPAC name of the first member of the series | Ethene | Ethyne | Ethane |
Type of reaction with chlorine | Addition | Addition | Substitution |
15. Fill in the blanks with the correct words from the brackets:
(a) Alkenes are the (i) ……… (analogous/homologous) series of (ii) …… (saturated/unsaturated) hydrocarbons. They differ from alkanes due to the presence of (iii) ………. (double/single) bonds. Alkenes mainly undergo (iv) ………. (additional/substitution) reactions.
Ans:
(i) Homologous
(ii) unsaturated hydrocarbons
(iii) Single
(iv) Addition
(b) The organic compound which undergoes substitution reaction is (v) ………. (C2H2, C2H4, C10H18, C2H6)
Ans: C2H6
(c) Draw the structural formulae of the two isomers of Butane. Give the correct IUPAC name of each isomer.
Ans:
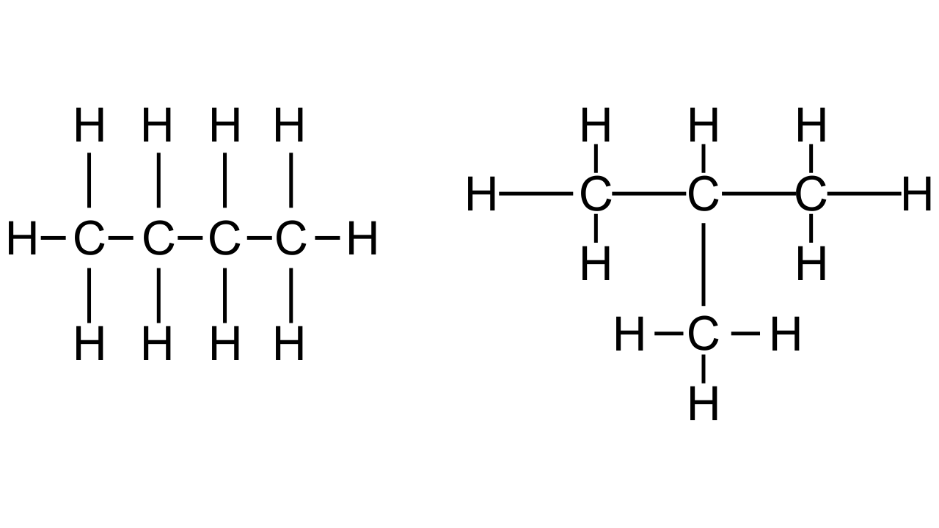
n-Butane and 2-Methylpropane
16. Name:
(a) The saturated hydrocarbon containing two carbon atoms.
Ans: Ethane
(b) An alcohol with three carbon atom.
Ans: Propan1-ol
(c) A triple bond hydrocarbon with two carbon atoms.
Ans: Ethyne
Exercise- 12B
1. State the sources of alkanes.
Ans: Oil and natural gas are the most prominent sources of alkanes.
2. Methane is a greenhouse gas. Comment
Ans: Natural gas is primarily composed of methane. It absorbs the earth's outgoing heat radiation and hence contributes to the greenhouse effect.
3. Give the general formula of the alkanes.
Ans: The general formula for alkanes is CnH2n+2, where n is the number of carbon atoms.
4. Draw the structures of isomers of:
(a) Butane
Ans:
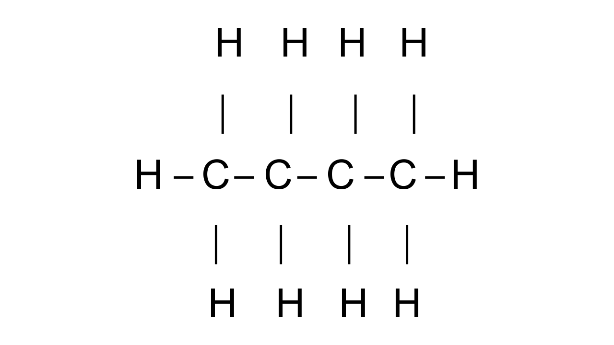
Common name: n-Butane
IUPAC name: Butane
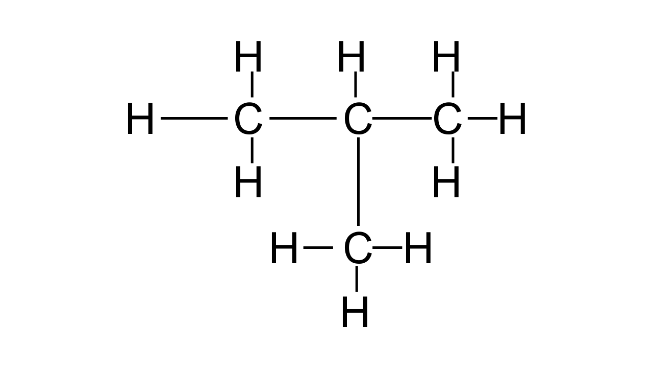
Common name: iso Butane
IUPAC name: 2-Methylpropane
(b) Pentane. Write the IUPAc and common name of these isomers.
Ans:
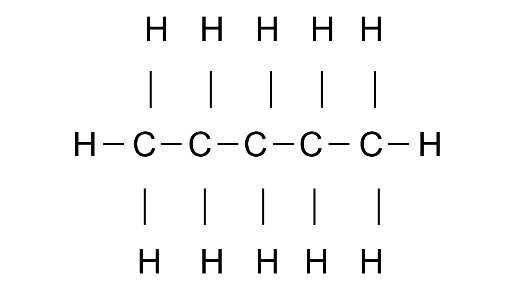
Common name: n-pentane
IUPAC name: Pentane
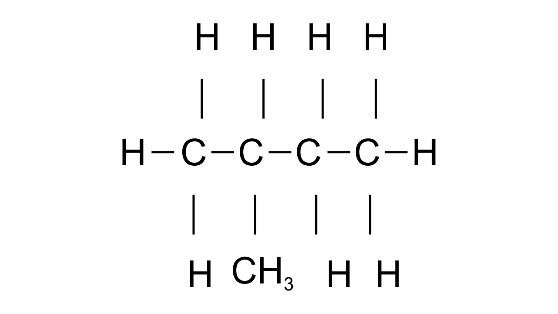
Common name: iso-pentane
IUPAC name: 2-Methylbutane
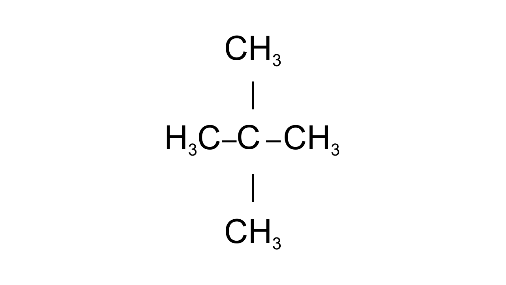
Common name: neo-pentane
IUPAC name: 2,2-Dimethylpropane
5. Write the
(a) molecular formula.
(b) Electron dot formula and
(c) Structural formula of methane and ethane.
Ans:
(a) Molecular formula
Methane- CH4
Ethane- C2H6
(b) Electron dot formula
Methane-
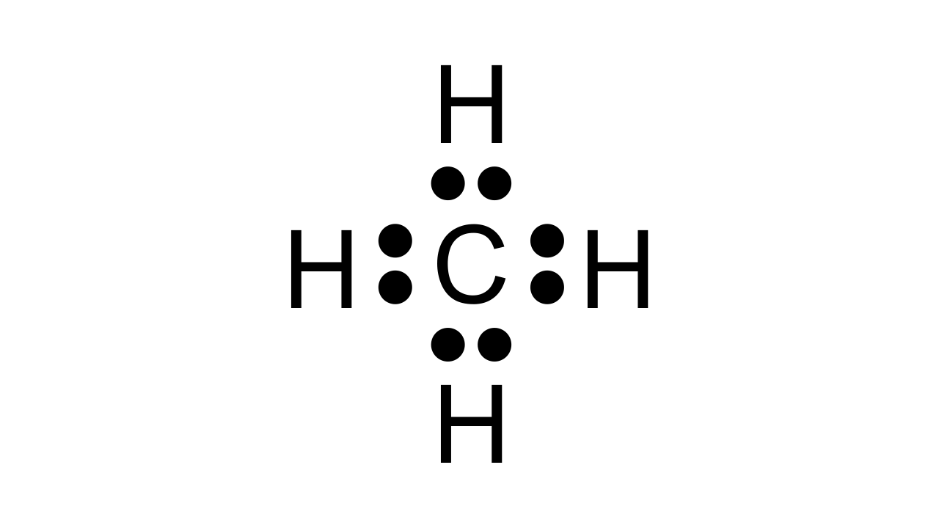
Ethane-

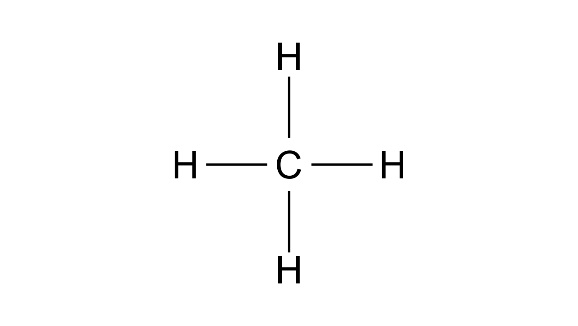
(c) Structural formula
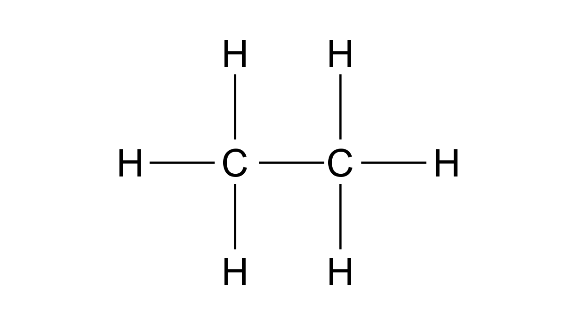
Methane-
Ethane-
6. How is:
(a) Methane and
(b) Ethane prepared in the laboratory?
Ans: (a) Laboratory preparation of methane
The gas produced when a mixture of sodium ethanoate and soda lime is cooked in a hard glass test tube is methane. Water is displaced downwards to collect it.
CH3COONa + NaOH + CaO → Na2CO3 + CH4 (at 3000C)
(b) Ethane prepared in laboratory
When a solution of sodium propionate and soda lime is heated in a boiling tube, ethane gas is produced. Water is also displaced downward, which collects it.
C2H5COONa + NaOH + CaO → Na2CO3 + C2H6 (at 3000C)
7. How are methane and ethane prepared from methyl iodide and ethyl bromide?
Ans: CH3I + 2[H] → CH4 + HI
C2H5Br + 2[H] → C2H6 + HBr
8. What is a substitution reaction? Give the reaction of chlorine with ethane and name the product formed.
Ans: A substitution reaction occurs when one of a molecule's atoms is replaced by another atom or group of atoms.
C2H6 + Cl2 → C2H5Cl + HCl
9. Name the compounds formed when methane burns in:
(a) Sufficient air
(b) insufficient air
Give a balanced equation.
Ans:
(a) The product formed will be carbon dioxide and water
CH4 + 2O2 → CO2 + 2H2O
(b) The product formed will be carbon monoxide and water
2CH4+ 3O2 (insufficient) → 2CO + 4H2O
10. Write the names and the formula of the products formed when:
(a) methane (b) ethane reacts with (i) chlorine (ii) bromine
Write the chemical equations:
Ans:
Methane reacts with chlorine
CH4 + Cl2 → CH3Cl + HCl
Methane reacts with Bromine
CH4 + Br2 → CH3Br + HBr
Ethane reacts with Chlorine
C2H6+ Cl2 → C2H5Cl + HCl
Ethane reacts with Bromine
C2H6 + Br2 → C2H5Br + HBr
11. Name the compound prepared from:
(a) Sodium propionate
(b) Methyl iodide and
(c) Ethyl bromide.
Write a balanced equation for the same.
Ans:
(a) C2H5COONa + NaOH + CaO → Na2CO3 + C2H6
(b) CH3I + 2[H] → CH4 + HI
(c) C2H5Br + 2[H] → C2H6 + HBr
12. Write the equation for the complete combustion of
(i) Methane
Ans: CH4 + 2O2 → CO2+ 2H2O
(ii) Ethane
Ans: 2C2H6 + 5O2 → 4CO2 + 2H2O
13. Convert:
(a) Methane into chloroform.
Ans: CH4+ Cl2 → CH3Cl + HCl
CH3Cl + Cl2 → CH2Cl2+ HCl
CH2Cl2 + Cl2 → CHCl3 + HCl
(b) Sodium acetate into methane
Ans: CH3COONa + NaOH → Na2CO3 + CO2
(c) Methyl iodide into ethane.
Ans: 2CH3I + 2Na + dry ether → C2H6 + NaI
(d) Methane to methyl alcohol
Ans: CH4 + O2 + → 2CH3OH (at 120 atm and 475K)
14. Give three uses of:
(a) Methane
Ans:
Methane is a carbon monoxide and hydrogen source.
It's used to make carbon tetrachloride from ethyne methane chloromethane.
It is used as a household fuel.
(b) Ethane
Ans:
It's used to make ethane ethanol and ethanol.
It produces ethyl chloride, which is utilised in the production of tetraethyllead.
It's also a source of energy.
15. Under what conditions does ethane get converted to:
(a) Ethyl alcohol
Ans: Ethyl alcohol is created when a combination of ethane and oxygen is compressed to around 120 atm pressure and pushed through copper tubes at 475K.
2C2H6 + O2 + → 2C2H5OH (at 120 atm and 475K)
(b) Acetaldehyde
Ans: Acetaldehyde is produced when a combination of ethane and oxygen is passed through heated molybdenum oxide.
C2H6 + O2 + MoO → CH3CHO + H2O
(c) Acetic acid
Ans: C2H5OH + O2 + Pt → CH3COOH + H2O
16. Using appropriate catalysts, ethane can be oxidised to an alcohol, an aldehyde and an acid. Name the alcohol, aldehyde and acid formed when ethane is oxidised.
Ans: Alcohol- Ethanol (C2H5OH)
Aldehyde- Ethanal (CH3CHO)
Acid- Ethanoic acid (CH3COOH)
Excercise- 12C
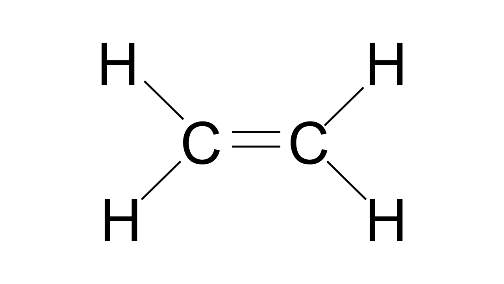
1. Write: (a) molecular formula (b) electron dot formula and (c) structural formula of ethene (ethylene).
Ans:
(a) C2H4
(b)
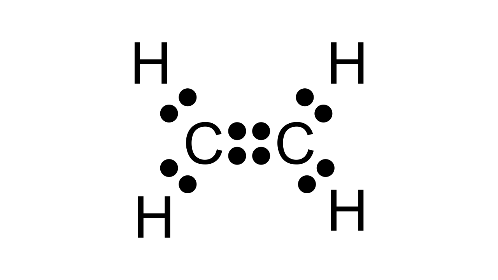
(c)
2. The molecules of alkene family are represented by a general formula CnH2n. Answer the following:
(a) What do n and 2n signify?
Ans: Alkanes have the generic formula CnH2n+2, where n is the number of carbon atoms in the molecule and 2n+2 is the number of hydrogen atoms.
(b) What is the name of alkene when n=4?
Ans: Butene
(c) What is the molecular formula of alkene when n=4?
Ans: C4H8
(d) What is the molecular formula of the alkene if there are ten H atoms in it?
Ans: C5H10
(e) What is the structural formula of the third member of the alkene family?
Ans: CH2=CH-CH3
(f) Write the molecular formula of lower and higher homologous of an alkene which contains four carbon atoms.
Ans: Lower homologous of alkene which contains four carbons is C3H6
Higher homologues of alkene which contain four carbons is C5H10
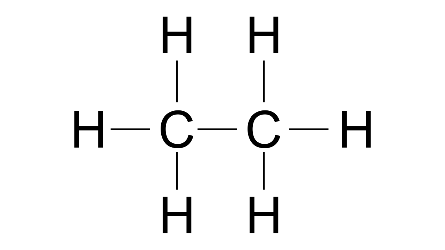
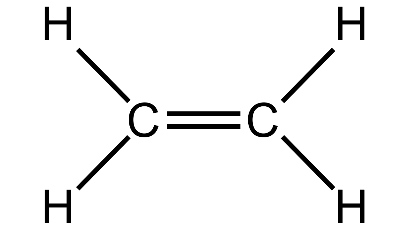
3. (a) Distinguish between the saturated hydrocarbon ethane and the unsaturated hydrocarbon ethene by drawing their structural formulae.
Ans:
Ethane-
Ethene-
(b) Draw the structures of isomers of butene and write their IUPAC names.
Ans: CH3-CH2-CH=CH2 But −1− ene
CH3-CH=CH-CH3 But −2− ene
CH2=C(CH3)-CH3− methyl propene
4. Give a balanced equation for the lab. Preparation of ethylene. How is the gas collected?
Ans: CH3-CH2OH + H2SO4 → CH3-CH2HSO4 + H2O
CH3-CH2HSO4 excess H2SO4 → CH2=CH2 + H2SO4 (at 1600C)
The gas is collected by downward displacement of water.
5. How is ethene prepared by:
(a) dehydrohalogenation reaction?
(b) dehydration reaction?
Give equations and name the products formed.
Ans: (a) Dehydrohalogenation is an elimination reaction in which a hydrogen halide is removed from a substrate.
C2H5Cl + alc. KOH → C2H4 + KCl + H2O
Ethene is formed as a product.
(b) C2H5OH + Al2O3 → C2H4 + H2O (at 3000C)
Ethene is formed as a product.
6. (a) Ethene when reacts with halogens (chlorine and bromine) from saturated products. Name them and write balanced equations.
Ans: CH2=CH2 + Cl2 → CH22(Cl)-CH2(Cl) - 1,2-Dichloroethane
CH2=CH2 + Br2 → CH2(Br)-CH2(Br) - 1,2-Dibromoethane
(b) Give the conditions and the main product formed by hydrogenation of ethene.
Ans: When ethene and hydrogen travel through a finely divided catalyst, such as platinum or palladium at room temperature or nickel at 200o C, the two hydrogen atoms are added to the unsaturated molecule, resulting in a saturated one.
C2H4 + H2 + Ni → C2H6 (at 2000C)
7. Convert ethanol into ethene using
(a) Solid dehydrating agent
(b) hot conc. H2SO4?
Give only balanced equations.
Ans: (a) C2H5OH + Al2O3 → C2H4 + H2O (at 3000C)
(b) CH3-CH2HSO4 excess H2SO4 → CH2=CH2 + H2SO4 (at 1600C)
8. Write the following properties of ethene:
(a) Physical state
Ans: Gaseous state.
(b) Odour
Ans: Sweetish odour
(c) Density as compared to air
Ans: Density lesser than air.
(d) Solubility
Ans: sparingly soluble in water but soluble in organic solvents.
9. How would you convert:
(a) ethyl bromide into ethene
Ans: C2H5Br + alc. KOH → C2H4 + KBr + H2O
(b) ethene into 1,2-dibromoethane
Ans: CH2=CH2 + Br2 → CH2 (Br)-CH2(Br)
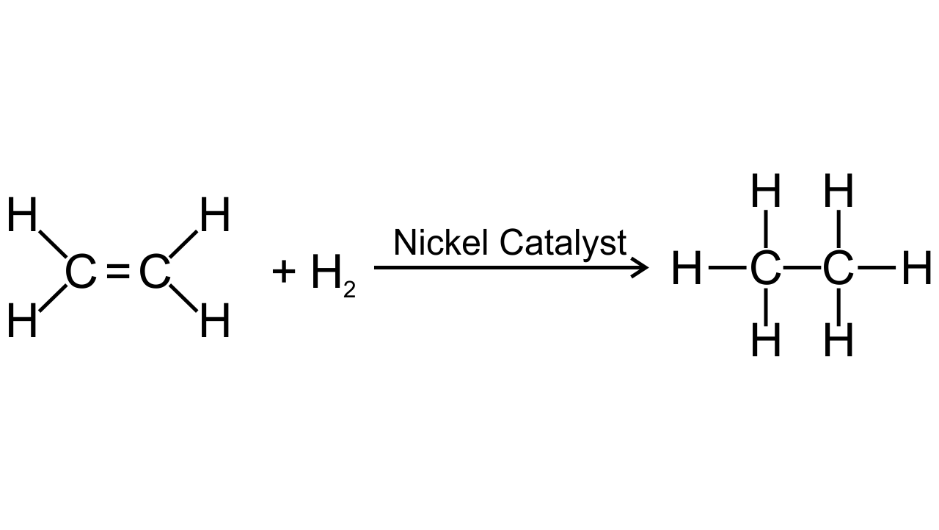
(c) ethene into ethane
Ans:
10. Give balanced equation when:
(a) ethene is burnt in excess of oxygen.
Ans: C2H4 + 3O2 → 2CO2+ 2H2O + heat
(b) ethene reacts with chlorine gas.
Ans: CH2=CH2 + Cl2 → CH2(Cl)-CH2(Cl)
(c) ethene combines with hydrogen chloride.
Ans: CH2=CH2 + HCl → CH3-CH2Cl
(d) a mixture of ethene and hydrogen is passed over nickel at 2000C.
Ans: C2H4 + H2 + Ni → C2H6 (at 2000C)
11. Give the formula and names of A, B,C and D in the following equations:
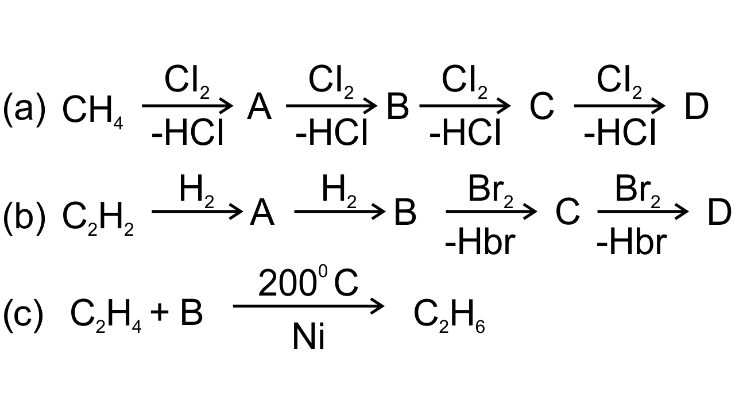
Ans: (a) A- CH3Cl (chloro methane)
B-CH2Cl2 (Di-chloromethane)
C-CHCl3 (tri-chloro methane)
D- CCl4 (Carbon tetrachloride)
(b) A- C2H4 (ethene)
B- C2H6 (ethane)
C-C2H5Br (bromo-ethane)
D- C2H4Br2 (1,2-Di-bromoethane)
(c) B- H2 (Hydrogen)
12. Write the name and formula of the product formed in each case below:
(a) C2H4 + Cl2 → ……….
Ans: 1,2-di-chloroethane (C2H4Cl2)
(b) C2H5I + KOH (alc.) → …………
Ans: Ethene (C2H4)
(c) H2C=CH2 + alk. KMnO4 → ……..
Ans: Ethane1,2di-ol (CH2(OH)-CH2(OH))
(d) H2C=CH2 + HBr→……..
Ans: Bromoethane (CH5Br)
13. What do you observe when ethene is passed through alkaline KMnO4 solution?
Ans: H2C=CH2 + alk. KMnO4 → CH2(OH)-CH2(OH)
Ethane1,2di-ol will be formed.
14. Name three compounds formed by ethene and give one use of each compound.
Ans: polythene, Ethanol, and Epoxyethane are formed by ethene.
Carrying bags are made of polythene.
Ethanol is a beginning element for a variety of different items, mostly cosmetics and toiletries. preparation.
Epoxyethane is a chemical that is used to make detergents.
Exercise- 12D
1. What are the sources for alkynes? Give the general formula of alkynes.
Ans: Alkynes can be found in natural gas and petroleum. Alkynes have the general formula: CnH2n-2
2. Give an example of isomers shown by triple bond hydrocarbons (alkynes) and write their IUPAC names.
Ans: Isomers shown by triple bond hydrocarbons is Butyne. Isomers are shown below:
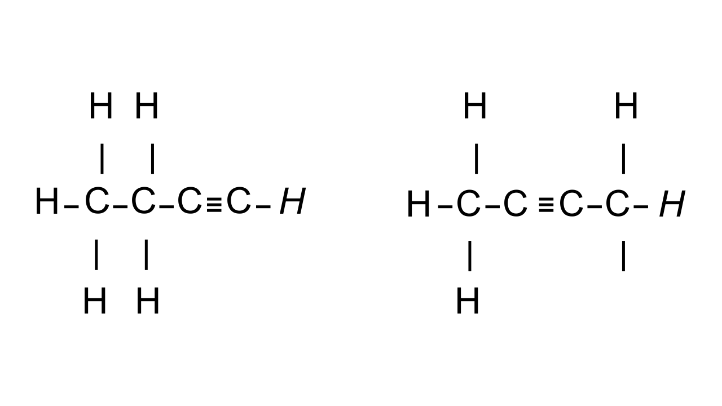
But1-yne But2-yne
3. How is ethyne prepared in the laboratory?
(a) Draw a diagram
Ans:
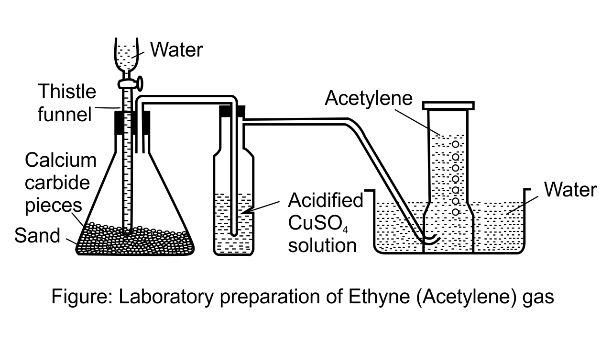
(b) Give an equation
Ans: CaC2 + 2H2O → Ca(OH)2 + C2H2
(c) How is pure dry gas collected?
Ans: Because the pure dry gas is insoluble in water, it is collected by water displacement downward.
4. Give the method of preparation of ethyne by: 1,2-dibromoethane.
Ans: CH2(Br)-CH2(Br) + Alc. KOH → C2H2 + 2KBr + 2H2O
5. Name the hydrocarbon which:
(a) is a tetrahedral molecule.
Ans: Methane is a tetrahedral molecule.
(b) Is a planar molecule.
Ans: Ethene is a planar molecule.
(c) Is a linear molecule.
Ans: Ethyne is a linear molecule.
(d) forms a red precipitate with ammoniacal solution of copper (I) chloride.
Ans: Ethyne forms a red precipitate with ammoniacal solution of copper (I) chloride.
(e) Is known as paraffin.
Ans: Saturated hydrocarbons or alkanes are known as paraffin.
(f) Is known as olefin.
Ans: Alkenes are known as olefin.
(g) a compound which will give ethyne (acetylene) gas when treated with water.
Ans: When calcium carbide is exposed to water, it produces acetylene gas.
6. Classify the following compounds as alkanes, alkenes and alkynes: C3H4, C3H8, C5H8, C3H6
Ans: C3H8 and C3H6 are alkanes. C3H4 and C5H8 are alkynes.
7. Give a chemical test to distinguish between
(a) Saturated and unsaturated compounds.
Ans: When bromine water is added to an unsaturated compound, bromine water is ejected in a red-brown colour. As a result, if an organic chemical decolorizes bromine water, it is an unsaturated hydrocarbon with a double or triple bond.
Alkanes, which are saturated hydrocarbons, do not decolorize bromine water.
(b) Ethane and ethene.
Ans: In two separate test tubes, dissolve ethane and ethene in carbon tetrachloride solution. Fill the two test tubes with bromine gas. If the colour of bromine gas changes, the gas is ethene; if the colour remains the same, the gas is ethane.
(c) Ethene (ethylene) and ethyne (acetylene).
Ans: Pass the gas through an ammoniacal cuprous chloride solution. Ethene will not form any ppt while ethyne will form red colour ppt.
8. Compound X is bubbled through bromine dissolved in CCl4;
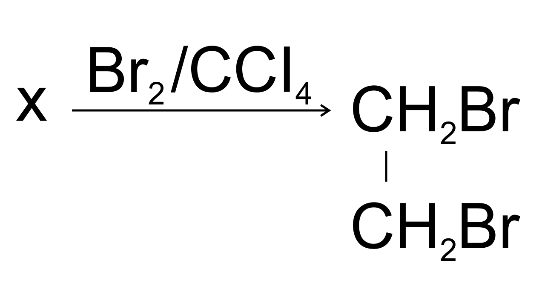
(a) Draw the structure of X.
Ans:
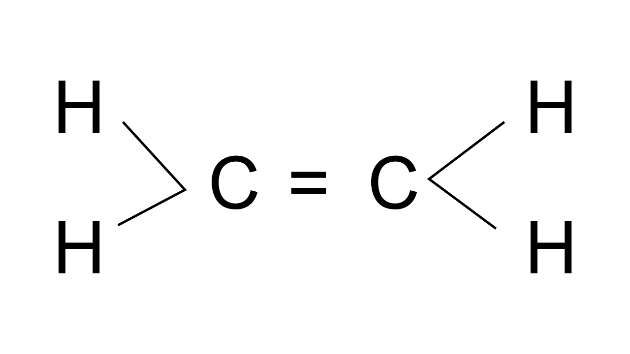
(b) State your observation during the reaction.
Ans: Bromine solution will be decolourised.
9. Give balanced equations for the following conversions:
(a) An alkene to an alkane
Ans: CH2 + CH2 + Ni + H2 → CH3CH3 (at 3000C)
(b) An alkene to an alcohol
Ans: CH2=CH2 + H2O → CH2CH2OH
(c) An alkyne to an alkene.
Ans: C2H2 + H2 + lindar’s reagent → C2H4
10. Name the products formed and write an equation when ethyne is added to the following in an inert solvent:
(a) chlorine
Ans: 1,2-dichloro ethene and 1,1,2,2 -tetrachloro ethane will be formed.
C2H2 + Cl2 → C2H2Cl2 + Cl2 → C2H2Cl4
(b) Bromine
Ans: ,2-dibromo ethene and 1,1,2,2 -tetrabromo ethane will be formed.
C2H2 + Br2 → C2H2Br2 + Br2 → C2H2Br4
(c) Iodine
Ans: 1,2-di-iodoethene will be formed.
C2H2 + I2 → C2H2I2
(d) Hydrogen
Ans: C2H2 + H2 + Ni → C2H6
(e) Excess of hydrochloric acid.
Ans: C2H2 + HCl → C2H2Cl2
11. Substitution reactions are characteristic reactions of ……… (alkynes/alkenes/alkanes).
Ans: Alkanes
12. (a) Write an equation for the laboratory preparation of
(i) An unsaturated hydrocarbon from calcium carbide.
Ans: CaC2 + H2O → C2H2 + Ca(OH)2
(ii) An alcohol from ethyl bromide.
Ans: CH3CH2Br + aq. KOH → CH3CH2OH
(b) What would you see, when ethyne is bubbled through a solution of bromine in carbon tetrachloride?
Ans: When bromine in carbon tetrachloride is introduced to ethyne, the colourless ethylene bromide is formed, and the orange colour of the bromine fades.
(c) Name the addition product formed between ethene and water.
Ans: Ethanol (C2H5OH)
13. Give reasons:
(a) Ethyne is more reactive than ethene.
Ans: Because of the presence of a triple bond between its two carbon atoms, ethyne is a more reactive molecule than ethene.
(b) Ethene is more reactive than ethane.
Ans: Because of the presence of a double bond between its two carbon atoms, ethane is a more reactive chemical than ethene.
(c) Hydrocarbons are excellent fuels.
Ans: Alkanes, for example, are hydrocarbons that react with oxygen to produce carbon dioxide and water vapour. Because alkanes are combustible, they make good fuels.
14. (a) Write the balanced equations:
(i) when butane is burnt in oxygen
Ans: C4H10 + 6O2 → 4CO2 + 5H2O
(ii) preparation of ethylene from ethyl alcohol.
Ans: C2H5OH + H2SO4 → C2H4 + H2O
(b) (i) Convert ethane to acetic acid
Ans: 2C2H6 + O2 + K hot tube → C2H5OH + Pt → CH3COOH (at 3000C)
(ii) Convert ethyne to ethane.
Ans: C2H2 + H2 + Ni → C2H6
15. (a) Write the equation for the preparation of carbon tetrachloride from methane.
Ans: CH4 + Cl2 → CH3Cl + HCl
CH3Cl + Cl2 → CH2Cl2 + HCl
CH2Cl2 + Cl2 → CHCl3 + HCl
CHCl3 + Cl2 → CCl4 + HCl
(b) Draw the structural formula of ethyne.
Ans:
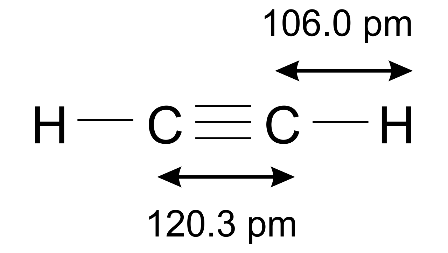
(c) How is the structure of alkynes different from that of alkenes?
Ans: Alkynes contain triple bonds while alkenes contain double bonds.
Exercise- 12E
1. (a) What are alcohols? State their sources.
Ans: Alcohols are alkane hydroxyl derivatives that are created by replacing one or more hydrogen atoms with an OH group. Methanol comes from the harmful distillation of wood, whereas ethanol comes from sugar fermentation.
(b) Give general formulae of monohydric alcohol.
Ans: CnH2n+1OH
2. Give the:
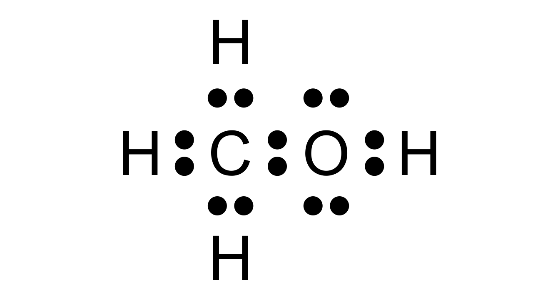
(a) Dot diagram of first member of alcohol.
Ans:
(b) Abbreviated formula of third member of alcohol.
Ans: CH3CH2CH2OH
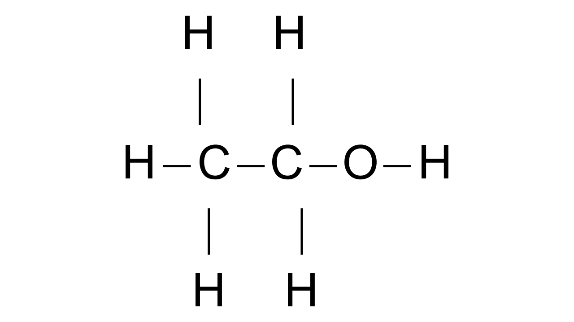
(c) Structure of second member of the alcohol group.
Ans:
(d) Structure of alcohol with 4 carbon atoms.
Ans:
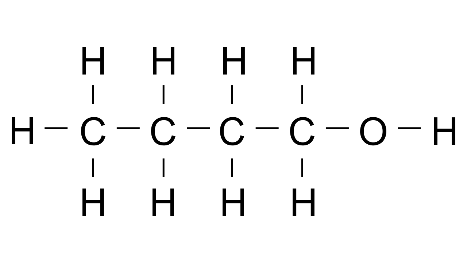
3. State the method of preparation of ethanol:
(a) By hydrolysis of ethane.
Ans: Ethyl hydrogen sulphate is created when concentrated sulphuric acid is added to ethene at a temperature of 80°C and a pressure of 30 atm. Hydrolysis of ethyl hydrogen sulphate with boiling water yields ethanol.
C2H4 + H2SO4 → C2H5HSO4
C2H5HSO4 + H2O → C2H5OH + H2SO4
(b) By hydrolysis of ethyl bromide.
Ans: The hydrolysis of alkyl halide with a hot dilute alkali can be used to make alcohols.
C2H5Cl + KOH → C2H5OH + KCl
4. Halo alkanes reacts with alkalies to produce alcohol. Give the equation for the preparation of second member of homologous series of alcohol. State under what condition the reaction occur.
Ans: Alcohol is produced when haloalkanes react with alkalis. Give the formula for the second member of the homologous series of alcohol. Indicate the circumstances in which the reaction occurs.
5. (a) How do the boiling point and melting point change in the homologous series of alcohols?
Ans: With increasing molecular mass, the melting and boiling points of subsequent members of the homologous series of alcohols rise.
(b) Name the product formed when ethanol reacts with acetic acid. Give an equation.
Ans: Ethyl acetate is generated when ethanol combines with acetic acid.
(c) What is the name given to this type of reaction?
Ans: Esterification is the name given to this process.
6. Complete and balance the following equations. State the conditions wherever necessary.
(a) \[\begin{align} & CH \\ & \text{ }|||+{{H}_{2}}\to \_\_\_\_\_+{{H}_{2}}\to \_\_\_\_ \\ & CH \\ \end{align}\]
Ans: C2H2 + H2 → C2H4 + H2 → C2H6
(b) C2H4 + Br2 → …………
Ans: C2H4 + Br2 → CH2(Br)-CH2(Br)
(c) C2H4 + HCl →………..
Ans: C2H4 + HCl → CH3CH2Cl
(d) CaC2+ H2O → ……….
Ans: CaC2 + H2O → Ca(OH)2 + C2H2
(e) C2H2 + Br2 → …………
Ans: C2H2 + Br2 → H(Br)C=C(Br)H
(f) C2H5OH + [O] + K2Cr2O7 →………
Ans: C2H5OH + [O] + K2Cr2O7 → CH3COOH
7. What is the effect of ethanol on human body?
Ans: Moderate ethanol intake lowers stress levels and boosts emotions of happiness and well-being, as well as lowering the risk of coronary heart disease. Heavy drinking, on the other hand, can lead to addiction and raise the risk of all forms of harm and trauma.
8. How are the following obtained:
(a) Absolute alcohol
Ans: By distilling wet alcohol with benzene, absolute alcohol can be obtained. The mixture of water and benzene distils away, leaving behind anhydrous alcohol.
(b) Spurious alcohol
Ans: It's made through faulty distillation. It's a blend of alcohol with a lot of methanol in it.
(c) Methylated spirit
Ans: Ethyl alcohol is mixed with 5% methyl alcohol, a coloured dye, and some pyridine to make denatured alcohol.
9. Name the products formed and give appropriate chemical equations for the following:
(a) Sodium reacting with ethyl alcohol.
Ans: When sodium interacts with ethyl alcohol, hydrogen is produced, and sodium ethoxide is formed.
C2H5OH + 2Na → 2C2H5ONa + H2
(b) Ethanol oxidised by acidified potassium dichromate.
Ans: Alcohols are oxidised and transformed to ethanal, which is then turned to acetic acid.
C2H5OH + [O] → CH3CHO
CH3CHO + [O] → CH3COOH
10. Give the trivial (common) names and the IUPAC names of the following:
(a) C3H6
Ans: Common name: Propylene
IUPAC: Propane
(b) C2H4
Ans: Common name: ethylene
IUPAC name: ethene
(c) C2H2
Ans: Common name: acetylene
IUPAC name: ethyne
(d) CH3OH
Ans: Common name: methyl alcohol
IUPAC name:methanol
(e) C2H5OH
Ans: Common name: ethyl alcohol
IUPAC name: ethanol
11. Ethanol can be oxidised to ethanoic acid. Write the equation and name the oxidising agent.
Ans: CH3CH2OH + [O] + K2Cr2O7 → CH3COOH + H2O
12. Name an organic compound which is:
(a) Used for illuminating country houses.
Ans: Ethyne
(b) Used for making a household plastic material.
Ans: Ethyne
(c) Called ‘wood spirit’.
Ans: Methanol
(d) Poisonous and contain OH group.
Ans: Methanol
(e) Consumed as a drink.
Ans: Ethanol
(f) Made from water gas.
Ans: Methanol
(g) Solvent for gums and resins.
Ans: Ethanol
(h) Dehydrated to produced ethene.
Ans: Ethanol
13. Ethanol can be converted into ethene which can be changed into ethane. Choose the correct word or phrase from the brackets to complete the following sentences.
(a) The conversion of ethanol into ethene is an example of………. (dehydration, dehydrogenation).
Ans: Dehydration reaction.
(b) Converting ethanol into ethene requires the use of ……… (conc. HCl, conc. HNO3, conc. H2SO4).
Ans: Conc. H2SO4
(c) The conversion of ethene into ethane is an example of……. (hydration, hydrogenation).
Ans: Hydration reaction.
(d) The catalyst used in the conversion of ethene into ethane is commonly …………. (iron, nickel, cobalt).
Ans: Nickel
14. Write the equations for the following lab, preparations:
(a) Ethane from sodium propionate.
Ans: C2H5COONa + NaOH → Na2CO3 + C2H6
(b) Ethene from idoethane.
Ans: C2H5I + alc. KOH→ C2H4 + KI + H2O
(c) Ethyne from calcium carbide.
Ans: CaC2 + H2O → Ca(OH)2 + C2H2
(d) Methanol from idomethane.
Ans: CH3I + NaOH → CH3OH + NaI
15. Name the compound prepared by each of the following reactions:
(i) C2H5COONa + NaOH →
Ans: C2H5COONa + NaOH → Na2CO3 + C2H6
(ii) CH3I + 2H →
Ans: CH3I + 2H → CH4 + HI
(iii) C2H5Br + KOH (alcoholic solution)→
Ans: C2H5Br + KOH (alcoholic solution)→ C2H4 + KBr + H2O
(iv) CO + 2H2 (Zinc oxide catalyst)→
Ans: CO + 2H2 (Zinc oxide catalyst)→ CH3OH
(v) CaC2 + 2H2O→
Ans: CaC2 + 2H2O→ Ca(OH)2 + C2H2
16. Write the equations for the following reactions:
(a) Calcium carbide and water.
Ans: CaC2 + H2O → Ca(OH)2 + C2H2
(b) Ethene and water (steam).
Ans: C2H4 + H2O → C2H5OH
(c) Bromoethane and an aqueous solution of sodium hydroxide.
Ans: C2H5Br + NaOH → C2H5OH + NaBr
Exercise-12F
1. What are carboxylic acids? Give their general formula
Ans: Carboxylic acids are found in abundance. Amino acids and fatty acids are two important examples. The carboxylate anion is formed when a carboxylic acid is deprotonated. CnH2n+1COOH is the general formula for carboxylic acids.
2. Write the names of:
(a) First three members of carboxylic acid series.
Ans: Formic acid, ethanoic acid, and propanoic acid are three members of the carboxylic acid series.
(b) Three compounds which can be oxidised directly, or in stages to produce acetic acid.
Ans: Ethanol, Acetylene, and Ethanal are three chemicals that can be oxidised directly or in steps to create acetic acid.
3. (a) Give the structural formulae of acetic acid.
Ans:
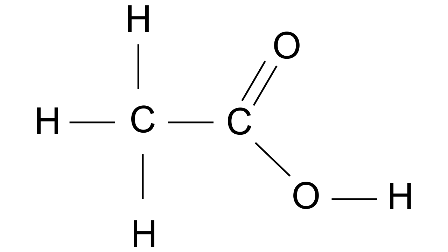
(b) IUPAC name of acetic acid.
Ans: Ethanoic acid
(c) What is glacial acetic acid?
Ans: Glacial acetic acid is pure acetic acid. Because when it cools, it crystallises into ice.
4. Vinegar is greyish in colour with a particular taste. Explain.
Ans: Vinegar, often known as Sirka, is a diluted acetic acid solution. It has a greyish appearance due to the presence of colouring matter, and it has the typical taste and flavour due to the presence of other organic acids and organic compounds.
5. Complete:
(a) Vinegar is prepared by the bacterial oxidation of………… .
Ans: Ethanol
(b) The organic acid present in vinegar is ………. .
Ans: Acetic acid
(c) The next higher homologue of ethanoic acid is ……….. .
Ans: Propanoic acid
6. How is acetic acid prepared from
(a) Ethanol
Ans: It's made by oxidising ethanol with acidified potassium dichromate in the lab.
C2H5OH + K2Cr2O7 →CH3COOH
(b) Acetylene
Ans: Acetylene is transformed to acetaldehyde first by passing it through a 40 percent H2SO4 solution at 60°C in the presence of 1% HgSO4. At 70°C, acetaldehyde is oxidised to acetic acid in the presence of manganous acetate as a catalyst.
C2H2 + H2O + dil.H2SO4 + HgSO4 → CH3CHO
CH3CHO + O → 2CH3COOH
7. What do you notice when acetic acid reacts with
(a) Litmus
Ans: When acetic acid reacts with litmus, the colour changes from blue to red.
(b) metals
Ans: Hydrogen is produced when acetic acid combines with metals.
(c) Alkalies
Ans: Salt is formed when acetic acid combines with alkalies.
(d) Alcohol
Ans: Esters are formed when acetic acid interacts with alcohols.
8. Acetic acid is a typical acid. Write one equation in each case for its reaction with
(a) A metal
Ans: 2CH3COOH + Zn → (CH3COO)2Zn + H2
(b) A base/alkali
Ans: CH3COOH + NaOH → CH3COONa + H2O
(c) A carbonate
Ans: 2CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2
(d) A bicarbonate
Ans: CH3COOH + NaHCO3 →CH3COONa + H2O + CO2
9. What do you observe when acetic acid is added to
(a) Sodium bicarbonate
Ans: Carbon dioxide is produced when acetic acid is introduced to sodium bicarbonate.
(b) Ethyl alcohol in the presence of sulphuric acid.
Ans: The ester (ethyl acetate) is generated when acetic acid is introduced to ethyl alcohol in the presence of sulphuric acid.
(c) Neutral FeCl3 solution?
Ans: Wine red hue is formed when acetic acid is added to neutral FeCl3.
10. Name:
(a) Compound formed when acetic acid and ethanol react together.
Ans: Ethyl acetate is formed when acetic acid and ethanol combine.
(b) Reducing agent used to convert acetic acid into ethanol.
Ans: Acetic acid is converted to ethanol using lithium aluminium hydride (LiAlH4).
(c) Substance used to change acetic acid to acetic anhydride.
Ans: Acetic anhydride is formed when phosphorus pentoxide (P2O5) is heated with acetic acid.
Miscellaneous
1. (a) Which of the following statements is wrong about alkanes?
(i) They are all saturated hydrocarbon.
(ii) They can undergo addition as well as substitution reaction.
(iii) They are almost non polar in nature.
(iv) On complete combustion give out carbon dioxide and water.
Ans: (ii) They can undergo only substitution reactions.
(b) The organic compound obtained as the end product of the fermentation of sugar solution is:
(i) Methanol
(ii) Ethanol
(iii) Ethane
(iv) Methanoic acid
Ans: Ethanol
(c) Find the odd one out and explain:
C3H8, C5H10, C2H6, CH4
Ans: C5H10 because it is not following the general alkane formula (CnH2n+2).
2. Give chemical equation for:
(a) The laboratory preparation of methane from sodium acetate.
Ans: CH3COONa + NaOH → CH4 + Na2CO3
(b) The reaction of one mole of ethene with one mole of chlorine gas.
Ans: C2H4 + Cl2 → CH2(Cl)-CH2(Cl)
(c) The preparation of ethyne from 1,2-dibromoethane.
Ans: CH2(Br)-CH2(Br) + 2KOH (alc.) → C2H2 + 2KBr + 2H2O
3. State how the following conversions can be carried out:
(a) Ethyl chloride to ethyl alcohol
Ans: C2H5Cl + aq. KOH → C2H5OH + KCl
(b) Ethyl chloride to ethene
Ans: C2H5Cl + alc. KOH → C2H4+ HCl
(c) Ethene to ethyl alcohol
Ans: C2H4 + H2O + H2SO4 → C2H5OH
(d) Ethyl alcohol to ethene
Ans: C2H5OH + H2SO4 → C2H4
4. (a) Define isomerism
Ans: The phenomenon of isomerism occurs when two or more compounds have the same chemical formula but distinct chemical structures.
(b) Give the IUPAC name of the isomer C4H10 which has a branched chain.
Ans: 2-Methylpropane
5. A compound X when treated with an organic acid Y (having vinegar like smell) in the presence of the acid Z, forms a compound P which has a fruity smell.
(a) Identify X, Y and Z.
Ans: X- alcohol
Y- Carbonic acid
Z- Sulphuric acid
(b) Write structural formula of X and Y.
Ans: X= R-OH
Y= R-COOH
(c) What type of compound is P?
Ans: Ester
(d) Name the above reaction.
Ans: Esterification reaction
(e) If compound x and y both have 2 carbon atoms. Write the reaction.
Ans: C2H5OH + CH3COOH + conc. H2SO4 → CH3COOC2H5 + H2O
2010
(a) An organic compound undergoes addition reactions and gives a red colour precipitate with ammoniacal cuprous chloride. Therefore, the organic compound could be:
(i) Ethane
(ii) Ethene
(iii) Ethyne
(iv) Ethanol
Ans: With ammoniacal cuprous chloride, an organic compound undergoes an addition reaction, resulting in a red colour precipitate. As a result, Ethyne could be an organic substance.
(b) An organic weak acid is:
(i) Formic acid
(ii) Sulphuric acid
(iii) Nitric acid
(iv) Hydrochloric acid
Ans: Formic acid
(c) The organic compound mixed with ethanol to make it spurious is
(i) Methanol
(ii) Methanoic acid
(iii) Methanal
(iv) Ethanoic acid
Ans: Methanol
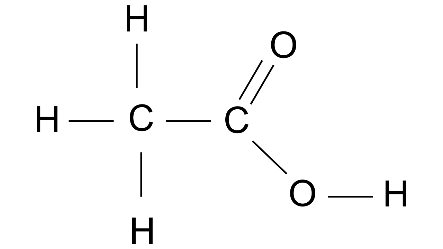
(d) Draw the structural formula for each of the following:
(i) Ethanoic acid
Ans:
(ii) But-2-yne
Ans: \[{{H}_{3}}C-C\equiv C-C{{H}_{3}}\]
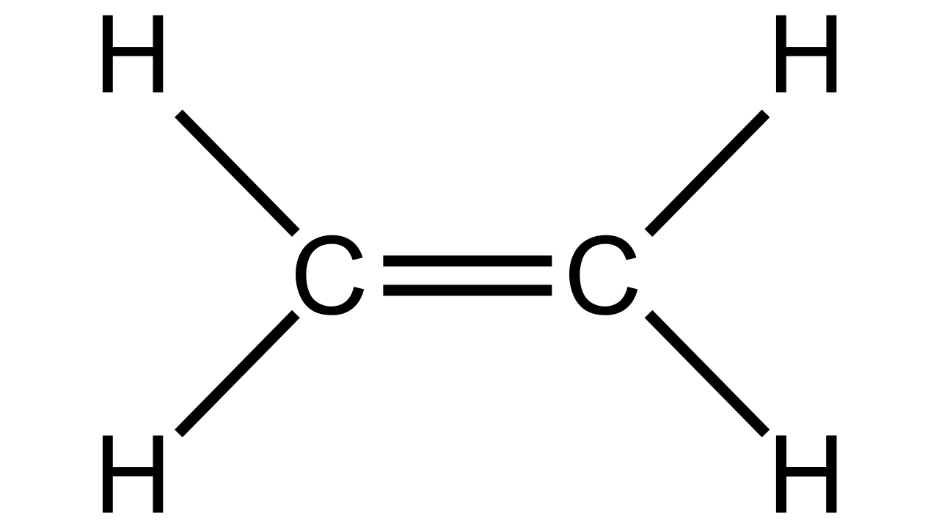
(e) Compound A is bubbled through bromine dissolved in carbon tetrachloride and the product is CH2Br-CH2Br.
(i) Draw the structural formula of A.
Ans:
(ii) What type of reaction has A undergone?
Ans: Addition reaction
(iii) What is your observation?
Ans: Compound A will decolourise the bromine water.
(iv) Name (not formula) the compound formed when steam reacts with A in the presence of phosphoric acid.
Ans: Ethanol
(v) What is procedure for converting the product of (e) (iv) back to A?
Ans: Dehydration- By heating it (ethanol) at 170 degrees Celsius with strong sulphuric acid.
2011
(a) The functional group present in acetic acid is:
(i) Ketonic >C=O
(ii) Hydroxyl -OH
(iii) Aldehyde -CHO
(iv) Carboxyl -COOH
Ans: Carboxyl -COOH
(b) The unsaturated hydrocarbons undergo:
(i) A substitution reaction
(ii) An oxidation reaction
(iii) An addition reaction
(iv) None of the above
Ans: Addition reaction
(c) The number of C-H bonds in ethane molecule are:
(i) Four
(ii) Six
(iii) Eight
(iv) Ten
Ans: Six
(d) Choose the correct word/phrase from within the brackets to complete the following sentences:
(i) The catalyst used for conversion of ethene to ethane is commonly …………. (nickel/iron/cobalt)
Ans: Nickel
(ii) When acetaldehyde is oxidized with acidified potassium dichromate, it forms………… (ester/ethanol/acetic acid)
Ans: acetic acid
(iii) Ethanoic acid reacts with ethanol in the presence of concentrated H2SO4, so as to form a compound and water. The chemical reaction which takes place is called………….. (dehydration/hydrogenation/esterification)
Ans: Esterification
(iv) Write the equation for the reaction taking place between 1,2-dibromoethane and alcoholic potassium hydroxide.
Ans:
(v) The product formed when ethene gas reacts with water in the presence of sulphuric acid………….(ethanol/ethanal/ethanoic acid)
Ans: Ethanol
(e) Write balanced chemical equations for the following:
(i) Monochloro ethane is hydrolysed with aqueous KOH.
Ans: CH3Cl + aq.KOH → CH3OH + KCl
(ii) A mixture of sodalime and sodium acetate is heated.
Ans: CH3COONa + NaOH + CaO → CH4 + Na2CO3
(iii) Ethanol under high pressure and low temperature is treated with acidified potassium dichromate.
Ans: C2H5OH + K2Cr2O7 → CH3COOH
(iv) Water is added to calcium carbide.
Ans: CaC2 + H2O → Ca(OH)2+ C2H2
(v) Ethanol reacts with sodium at room temperature.
Ans: CH3OH + Na → CH3ONa + 2H2
2012
(a) Give the structural formula for the following:
(i) Methanoic acid
Ans:
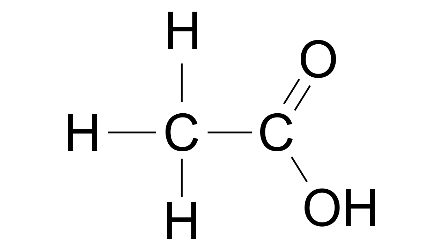
(ii) Ethanal
Ans:
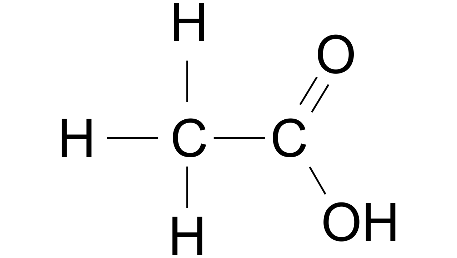
(iii) Ethyne
Ans: \[HC\equiv CH\]
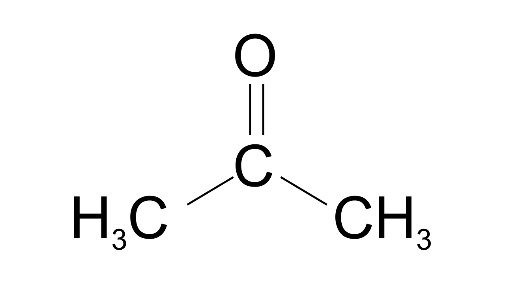
(iv) Acetone
Ans:
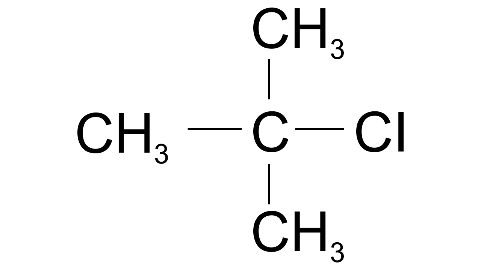
(v) 2-methyl propane
Ans:
(b) From the following organic compounds given below, choose one compound in each case which relates to the description (i) to (iv):
(ethyne, ethanol,acetic acid, ethene, methane).
(i) An unsaturated hydrocarbon used for welding purposes.
Ans: Ethyne
(ii) An organic compound whose functional group is carboxyl.
Ans: Acetic acid
(iii) A hydrocarbon which on catalytic hydrogenation gives a saturated hydrocarbon.
Ans: Ethene
(iv) An organic compound used as a thermometric liquid.
Ans: Ethanol
(C) (i) Why is pure acetic acid known as glacial acetic acid ?
Ans: Because it solidifies just below room temperature, pure ethanoic acid is referred to as glacial ethanoic acid.
(ii) Give a chemical equation for the reaction between ethyl alcohol and acetic acid.
Ans: CH3COOH + C2H5OH + conc. H2SO4 → CH3COOC2H5 + H2O
2013
(a) (i) Give a chemical test to distinguish ethene gas and ethane gas.
Ans: In two separate test tubes, dissolve ethane and ethene in carbon tetrachloride solution. Fill the two test tubes with bromine gas. If the colour of bromine gas changes, the gas is ethene; if the colour remains the same, the gas is ethane.
(ii) Identify the statement that is incorrect about alkanes:
(A) They are hydrocarbons.
(B) There is a single covalent bond between carbon and hydrogen.
(C) They can undergo both substitution as well as addition reactions.
(D) On complete combustion they produce carbon dioxide and water.
Ans: C is incorrect. Alkanes can undergo only substitution reactions.
(b) Give the structural formula for the following:
(i) An isomer of n-butane
Ans:
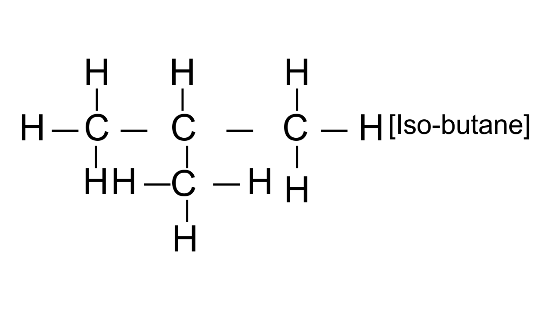
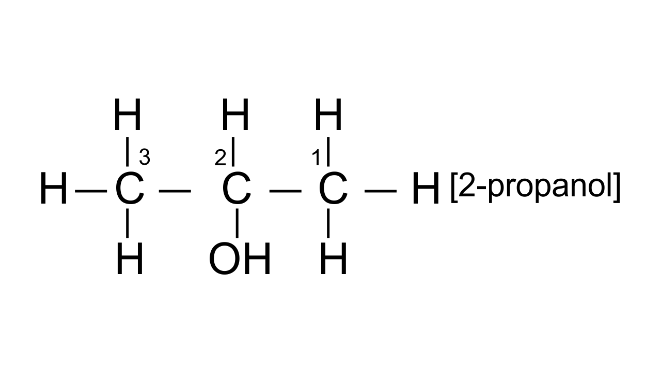
(ii) 2-propanol
Ans:
2014
(a) The IUPAC name of the acetylene is:
(i) Propane
(ii) Propyne
(iii) ethene
(iv) ethyne
Ans: Ethyne
(b) Name hydrocarbons containing >C=O functional group.
Ans: Carbonyl compounds.
(c) Give preparation of ethane from sodium propionate.
Ans: C2H5COONa + NaOH + CaO → C2H6 + Na2CO3
(d) Distinguish ethane and ethene (using alkaline potassium permanganate solution).
Ans: The purple colour of potassium permanganate does not fade in ethane, whereas the purple colour fades in ethene.
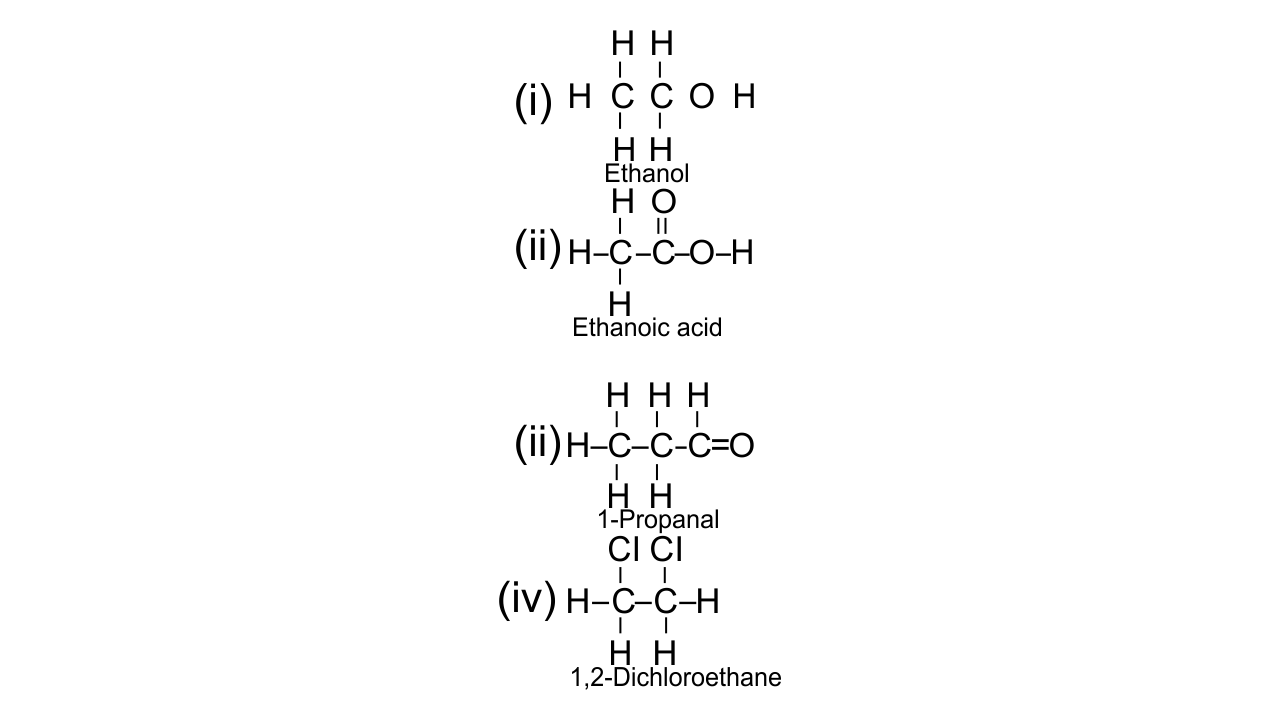
(e) Give the structural formula of the following:
(i) ethanol
(ii) 1-propanal
(iii) ethanoic acid
(iv) 1,2, dichloroethane
Ans:
(f) Give preparation of ethanol from monochloroethane and aq. Sodium hydroxide.
Ans: C2H5Cl + NaOH → C2H5OH + NaCl
2015
(a) Give balanced chemical equations for the following conversions:
(i) ethanoic acid to ethyl ethanoate.
Ans: CH3COOH + C2H5OH + conc. H2SO4 → CH3COOC2H5 + H2O
(ii) Calcium carbide to ethyne
Ans: CaC2+ 2H2O → Ca(OH)2 + C2H2
(iii) Sodium ethanoate to methane.
Ans: CH3COONa + NaOH → Na2CO3 + CH4
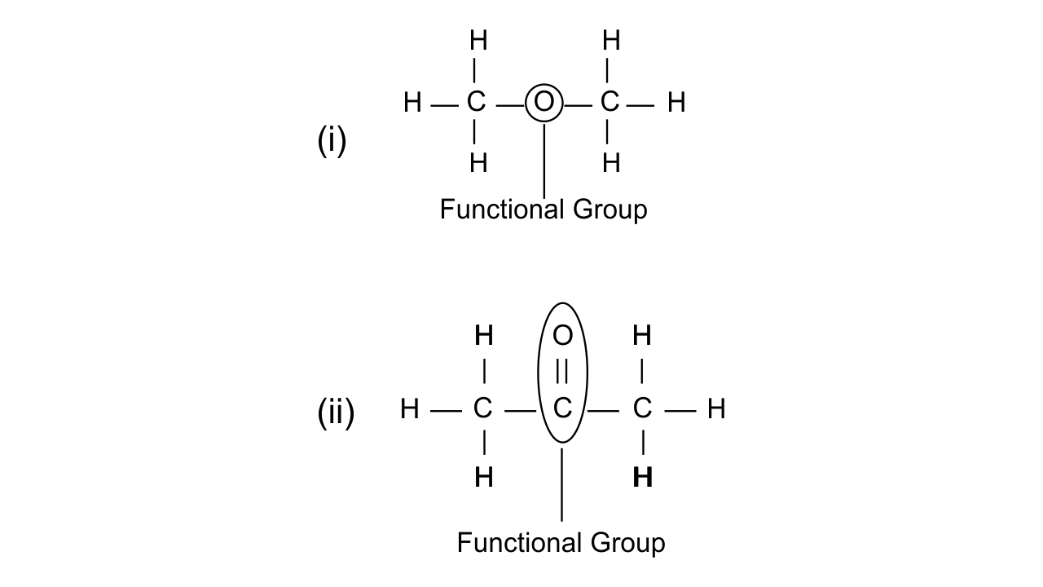
(b) Using their structural formula identify the functional group by circling them:
(i) Dimethyl ether
(ii) Propanone
Ans:
(c) Name the following:
(i) Process by which ethane is obtained from ethene
Ans: Hydrogenation process.
(ii) A hydrocarbon which contributes towards the greenhouse effect.
Ans: Methane
(iii) Distinctive reaction that takes place when ethanol is treated with acetic acid.
Ans: Esterification reaction
(iv) The property of elements by virtue of which atoms of the element can link to each other in the form of a long chain or ring structure.
Ans: Catenation
(v) Reaction when an alkyl halide is treated with alcoholic potassium hydroxide.
Ans: C2H6Cl + alc. KOH → C2H4
(d) Hydrocarbon which is a greenhouse gas is:
A. Acetylene
B. Ethylene
C. Ethane
D. Methane
Ans: Methane
Chapter 12 of the Class 10 Chemistry, ICSE Board (Concise – Selina Publishers).
Chapter 12 of the Class 10 Chemistry textbook is “Organic Chemistry”. The chapter serves as an introduction for the students to the world of Organic Chemistry. Now before diving into the contents of the chapter, let us first take a quick look at the Syllabus for Chapter 12, “Organic Chemistry”.
Syllabus:
The first topic is Introduction to Organic Chemistry.
Structure and Isomerism
Homologous Series
Simple nomenclature
Hydrocarbons: Alkanes, alkenes.
Alcohols: Ethanol – Preparation, Properties, and uses
Carboxylic acids (Aliphatic – MonoCarboxylic Acid): Acetic acid – Preparation, properties, and uses of Acetic acid, and preparation of the same from Ethyl alcohol
Content of the Textbook.
The content is divided into six parts - A, B, C, D, E, F. At the end of each part exercise for the same part follows. Solutions for all these parts are available at Vedantu.
The first part - 12 A, is “Organic Compounds', following are the topics covered under this part.
Introduction
As the heading of the topic suggests, this section introduces the topic of “Organic Compounds”.
Organic Compounds:
This heading discusses the source of organic compounds such as Plants, Coal, Animals, etc. Then there is a table showing a comparison between the organic and inorganic compounds. And finally, it ends with the small topic of Application of Organic Chemistry.
Unique Nature of Carbon Atoms.
Unique properties of Carbon are discussed here like the Tetravalency of the carbon atom and Catenation. The catenation is further discussed in two small topics: -
First is, Formation of straight, branched, and cyclic chains of carbon atoms.
Then, the Formation of single, double, and triple covalent bond
Furthermore, the features of Covalent bonding are discussed here as well.
Types of Organic Compounds:
As the heading suggests, it discusses the types of Organic compounds.
Hydrocarbons:
Definition and the classification of Hydrocarbons are explained here.
ALKYL Group:
The 6th section discusses how the ALKYL group can be obtained.
Functional Group.
This small section defines the Functional group and then explains whether the Alkanes contains any functional group. After that, three characteristics of functional groups are discussed.
Structure:
The structural formula of Hydrocarbons is explained here.
ISOMERS:
It explains what ISOMERS are.
Homologous Series.
It discusses the meaning of the Homologous Series, and the Characteristics as well as the significance of the same.
Nomenclature.
It explains the system of naming the organic compounds and the rules for doing so. After that many examples are followed.
Writing Structural Formulas from the IUPAC Name.
It explains the steps to follow for writing structural formulas from the IUPAC name.
The second part - 12 B, is Hydrocarbons: Alkanes. It explains the following concepts.
Alkanes.
Sources of Alkanes.
Methane and Ethane.
Methods of preparation of Methane and Ethane.
The third part - 12 C, is Hydrocarbons: Alkenes.
It explains,
Alkenes.
Ethene:- Structure, and preparation
Properties of Alkenes.
Uses of Ethane.
The fourth part - 12 D, is Hydrocarbons: Alkynes.
It explains,
Alkynes.
Ethyne.
Structure of Ethyne.
Laboratory preparation of ethyne.
Properties of acetylene.
Uses of ethyne.
The fifth part - 12 E, is Alcohols.
It Explains,
Alcohols.
Ethanol.
The last part - 12 F, is Carboxylic Acids.
It explains,
Carboxylic Acids.
Acetic Acid CH3COOH.
As said the exercise for each part is separate and at the end of each part the exercise is given. Many questions are asked in all the exercises combined. And all the exercises are solved by the expert teacher of Vedantu and the same is available for a free PDF download at Vedantu.
FAQs on Organic Chemistry Solutions for ICSE Board Class 10 Science (Concise - Selina Publishers)
1. The chapter is so lengthy how can I tackle it?
Yes, the chapter is for sure a lengthy one, but if done in an organized manner, you can easily tackle it. First of all, you have to make a mindset that “you can do it”, next, make a plan and then stick to it. The chapter always seems lengthy at first but try to deal with one topic at a time this way it will become easier for you. When the climbers climb the mountains they only take one step at a time, in that manner, they climb thousands of steps.
2. I have written the IUPAC name of all the Organic Compounds asked in the very first question of Exercise 12 A, how can I make sure my answers are correct?
First of all, you have done a great job. Now, you can visit the Vedantu website and match your answers with the solutions. And not only the first question, but the whole solution for ICSE Class 10 Chemistry Chapter 12 “Organic Chemistry” is available at Vedantu. So, you can check the whole solution here at Vedantu. Also, it is a really good habit to check your answers with that provided by the Vedantu and make sure that you are advancing on the right way.
3. Is it really important to solve all the questions of the exercise?
Yes, it is important to solve all the questions. Understandably, there are many questions but Vedantu makes it easy for you because you can find the complete solution for Class 10 Chemistry Chapter 12 at Vedantu. Solving the exercise will improve your brain muscles, and at the end of the day, you will learn, understand and remember all the things of the chapters, so that the questions in the exam will become easy for you to deal with.
4. Where can I find the explanations of the solution?
The downloadable PDF file that Vedantu provides for free, includes an explanation along with the solution. Our expert teachers have explained in such a manner that it will be extremely easy for you to comprehend and understand. The solution provided by the Vedantu for organic chemistry which is the 12th chapter of the Class 10 ICSE board, explains everything, including multiple-choice type questions if given in the exercise, therefore be sure to download the solution provided by the Vedantu.
5. Why should I follow Vedantu Solutions for Class 10, Chapter 12 of Chemistry?
The solutions for the Class 10, Chemistry chapter 12 “organic chemistry” are prepared for you by the expert teachers and explained by them in really easy and understandable language. Therefore, the solution provided by the Vedantu will make it easy for you to understand “Organic Chemistry”. As said earlier, our teachers, who are experts in the subjects, provide solutions in a manner that will become very much easy for the students to follow, and to understand, hence you should follow the solutions provided by Vedantu.






































