Vedantu’s Study of Compounds-Nitric Acid Solutions for ICSE Class 10 Chemistry
Free download of step by step solutions for class 10 Chemistry chapter 10 - Study of Compounds-Nitric Acid of ICSE Board (Concise - Selina Publishers). All exercise questions are solved & explained by expert teacher and as per ICSE board guidelines.
ICSE Selina Solutions for Class 10 Chemistry
Intex Questions
1. What is:
a) Aqua Fortis:
Ans: Nitric acid is called aqua Fortis. Aqua means water and Fortis means strong, it is so-called because nitric acid is a colorless liquid that is highly corrosive. It reacts with nearly all metals (except gold) and converts them to corresponding soluble nitrates. It can even dissolve silver which is not dissolved in any other acid. It has a molecular formula as HNO3.
b) Aqua Regia:
Ans: Aqua regia (Latin for royal water) is a freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid mixed in the ratio of 3 : 1 by volume. It can dissolve gold and platinum. The acids combine to evolve nascent chlorine (Cl). Nascent chlorine is very reactive and reacts with both gold and platinum. Hence, gold and platinum are soluble in it
3HCl + HNO3 \[\to \] NOCl+2 H2O +2[Cl]
Nitrosyl Chloride Nascent
Au + 3 [Cl] \[\to \] AuCl3
Pt + 4 [Cl] \[\to \] PtCl4
Aqua regia helps to remove the dull layer from the surface of gold giving it a shining look.
c) Fixation of Nitrogen:
Ans: The process of converting atmospheric nitrogen (N2) into useful nitrogenous compounds such as ammonia etc. is known as fixation of nitrogen. The triple bond of nitrogen breaks and nitrogen combines with hydrogen and oxygen to form the nitrogenous compounds.
2. During thunderstorms, rain water contains nitric acid. Explain with reactions.
Ans: During thunderstorms, the nitrogen present in the atmosphere reacts with oxygen to form nitric oxide when lightning discharges.
N2+O2 \[\to \] 2 NO
Nitric oxide
Nitric oxide is further oxidized to nitrogen dioxide.
2 NO + O2 \[\to \] 2 NO2
Nitrogen Dioxide
The nitrogen dioxide reacts with the atmospheric moisture or rain water in the presence of oxygen in air and forms nitric acid which is washed down during rain and combines with the salt present on the surface of the earth.
4 NO2 + 2 H2O + O2 + 4 HNO3
Nitric Acid
3. Ammonia is used in the Ostwald process
a. Give the source of reactants used in this process.
Ans: Dry ammonia (free from moisture) produced in Haber’s process and dry air free from carbon dioxide are the sources of reactants used in the Ostwald process.
b. Name the catalyst used in the process.
Ans: Platinum is used as a catalyst in the process. Platinum catalyses the ammonia in the presence of oxygen to nitric oxide, which is further oxidised to nitrogen dioxide.
4 NH3 + 5O2 \[\to \] 4 NO + 6 H2O + Heat
c) Name the oxidising agent used in this process.
Ans: Oxygen is the oxidising agent used in this process, which oxidises the nitric oxide to nitrogen dioxide.
2 NO + O2 \[\to \] 2 NO2
Nitric Oxide Nitrogen Dioxide
Nitrogen dioxide further undergoes hydrogenation in the presence of oxygen to form nitric acid.
d) What is the ratio of ammonia and air taken in this process?
Ans: The ratio of ammonia and air is taken in the process is 1:10. This means that 1 volume of ammonia will react with 10 volumes of air.
e) Why is quartz used in this process?
Ans: Quartz used in this process is acid-resistant and helps in the absorption of nitrogen dioxide in water in a uniform manner to form nitric oxide when packed in layers.
4. (a) Write a balanced chemical equation for the laboratory preparation of nitric acid.
Ans: The balanced chemical equation for the laboratory preparation of nitric acid is:
KNO3 + H2SO4 \[\to \] KHSO4 + HNO3
Potassium Nitrate Conc. Nitric Acid
In the laboratory, nitric acid is prepared by distilling conc. sulphuric acid with potassium nitrate, KNO3 (nitre) or sodium nitrate, NaNO3 (Chile saltpetre).
b) In the preparation of nitric acid from KNO3. Concentrated hydrochloric acid is not used in place of concentrated sulphuric acid. Explain why.
Ans: Concentrated hydrochloric acid cannot be used in place of conc. sulphuric acid in the preparation of nitric acid from KNO3 because hydrochloric acid is a volatile acid and hence nitric acid vapours will carry HCI vapours with it in the preparation of HNO3 and it will be difficult to get the nitric acid from the mixture.
c) Conc. nitric acid prepared in the laboratory is yellow in colour. Why? How is this colour removed?
Ans: Concentrated nitric acid prepared in the laboratory is yellow in color due to dissolution of reddish brown nitrogen dioxide gas in the acid. Nitric acid is unstable to heat and sunlight and thermally decompose to form nitrogen dioxide gas as shown below:
4 HNO3 \[\to \] 2 H2O + 4 NO2 + O2
The yellow color of the acid can be removed by bubbling the dry air or carbon dioxide, CO2 gas through the acid as it drives out the NO2 gas from the acid, which is further oxidised to form the colorless nitric acid. Also by adding excess water, nitrogen dioxide gas will dissolve in water and the yellow color of acid is removed and hence the colorless nitric acid is obtained.
d) Give reasons for the following:
In the laboratory preparation of nitric acid, the mixture of concentrated sulphuric acid and sodium nitrate should not be heated very strongly above 200°C.
Ans: In the laboratory preparation of nitric acid, the mixture of concentrated sulphuric acid and sodium nitrate should not be heated very strongly above 200°C because:
Sodium sulphate formed at high temperature forms a hard crust that sticks to the wall of the retort and is quite difficult to remove.
Glass apparatus may be damaged at higher temperatures.
At higher temperatures, the nitric acid also decomposes to give nitrogen dioxide.
5. (a) Nitric acid cannot be concentrated beyond 68% by the distillation of a dilute solution of HNO3. State the reason.
Ans: The mixture of nitric acid with water forms a constant boiling mixture which is 68% concentrated nitric acid. This mixture boils with uniform composition at a constant boiling point. At this temperature, the volatile nitric acid and the water vapour escape having uniform composition. So nitric acid cannot be concentrated beyond 68% by distillation of a dilute solution of HNO3.
(b) What is passive iron? How is passivity removed?
Ans: The iron becomes chemically inert or passive when dipped in concentrated nitric acid due to the formation of the thin layer of oxide Fe3O4 on its surface. This inert iron is known as passive iron. Passivity can be removed by heating the passive iron with a strong reducing agent or by rubbing the surface of the passive iron with the sandpaper.
6. Name the product formed when:
a. Carbon and conc. nitric acid is heated
Ans: The products formed when carbon and conc. nitric acid is heated are carbon dioxide, nitrogen dioxide and water.
C + 4 HNO 3 \[\to \] CO2 + 2 H2O + 4 NO2
b. Dilute HNO3 is added to copper.
Ans: The products formed when dilute HNO3 is added to copper are copper nitrate, nitric oxide and water.
3 Cu + 8 HNO 3 \[\to \] 3 Cu(NO3)2 + 4 H2O + 4 NO
7. Give two chemical equations for each of the following:
a) Reactions of nitric acid with non-metals.
Ans: Carbon and sulphur are the non-metals their reactions with nitric acid are as follows.
C + 4 HNO3 \[\to \] CO2 + 2 H2O + 4 NO2
S + 6 HNO3 \[\to \] H2SO4 + 2 H2O + 6 NO2
b) Nitric acid showing as acidic character.
Ans: Metal oxides behave as alkalis, react with nitric oxide and undergo a neutralisation reaction to form the corresponding soluble metal nitrates and water.
K2O + 2 HNO3 \[\to \] 2KNO3 + H2O
ZnO + 2 HNO3 \[\to \] Zn(NO3)2 + H2O
c) Nitric acid acting as oxidizing agent.
Ans: Nitric acid acts as a strong oxidizing agent and readily oxidises non-metals, metals, inorganic and organic compounds.
P4 + 20 HNO3 \[\to \] 4 H3PO4 + 4 H2O + 20 NO2
3 Zn + 8 HNO3 \[\to \] 3 Zn(NO3)2 + 4 H2O + 2 NO
8. Write balanced equations and name the products formed when:
(a) Sodium hydrogen carbonate is added to nitric acid.
Ans: Nitric acid reacts with carbonates and bicarbonates to give salt, water and carbon dioxide. Hence, when sodium hydrogen carbonate is added to nitric acid sodium nitrate, carbon dioxide and water are formed.
NaHCO3 + HNO3 \[\to \] NaNO3 + H2O + CO2
b) Cupric oxide reacts with dilute nitric acid
Ans: Cupric oxide is a metal oxide which behaves as alkali and undergo netralisation reaction when react with nitric acid. Hence, when cupric oxide reacts with dilute nitric acid, it forms copper nitrate salts, and water.
CuO + 2 HNO3 \[\to \] Cu(NO3)2 + H2O
c) Zinc reacts with dilute nitric acid
Ans: Zinc reacts with nitric acid to form zinc nitrate, nitric oxide and water.
3 Zn + 8 HNO3 \[\to \] 3 Zn(NO3)2 + 4 H2O + 2 NO
Nitric acid reacts with all metals except platinum and gold. The action of nitric acid and metals depends upon the concentration of nitric acid and temperature. Dilute nitric acid oxidises metals to their corresponding nitrates and evolves nitric oxide.
Concentrated nitric acid is heated
Ans: When concentrated nitric acid is heated it undergoes a decomposition reaction to form nitrogen dioxide gas, water and oxygen gas.
4 HNO3 \[\to \] 2 H2O + 4 NO2 + O2
9. How will you prepare the following from nitric acid?
(a) Sodium nitrate
Ans: Sodium hydroxide reacts with nitric acid to form sodium nitrate.
NaOH + HNO3 \[\to \] NaNO3 + H2O
Sodium Nitrate
(b) Copper nitrate
Ans: Copper oxide is a metallic oxide which behaves as basic oxide reacts with nitric acid and undergoes a neutralisation reaction to form copper nitrate and water.
CuO + 2 HNO3 \[\to \] Cu(NO3)2 + H2O
(c) Lead nitrate
Ans: Lead reacts with conc. nitric acid to form lead nitrate. Nitric acid reacts with metals to form metal nitrates and nitrogen dioxide.
Pb + 4 HNO3 \[\to \] Pb(NO3)2 + 2 H2O + 2 NO2
d) Magnesium nitrate
Ans: Dilute solution of nitric acid reacts with magnesium and manganese to give their nitrates and hydrogen gas. Hence, magnesium with dil. nitric acid forms magnesium nitrate and hydrogen gas is evolved.
Mg + 2 HNO3 \[\to \] Mg(NO3)2 + H2
(e) Ferric nitrate
Ans: Iron does not react with dilute nitric acid as it forms a layer of oxide over its surface, but it reacts with concentrated nitric acid to form ferric nitrate and liberates nitrogen dioxide gas.
Fe + 6 HNO3 \[\to \] Fe(NO3)3 + 3 H2O + 3 NO2
(f) Aqua regia
Ans: Aqua regia (Latin for royal water) is a freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid mixed in the ratio of 3:1 by volume. It can dissolve gold and platinum. The acids combine to evolve nascent chlorine (Cl).
3 HCl + HNO3 \[\to \] NOCl + 2 H2O + 2 [Cl]
Nitrosyl Chloride Nascent
10. Write the equation for the following conversions A, B. C and D.
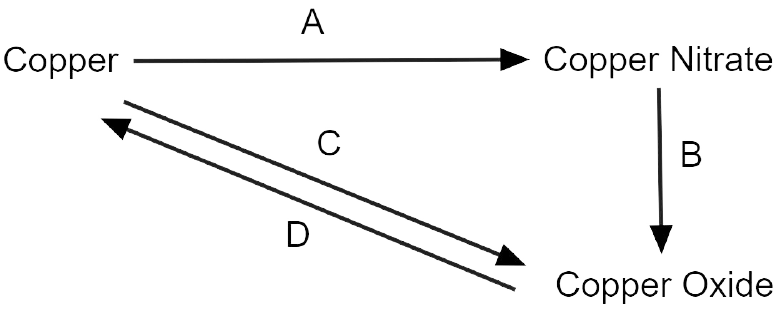
Ans:
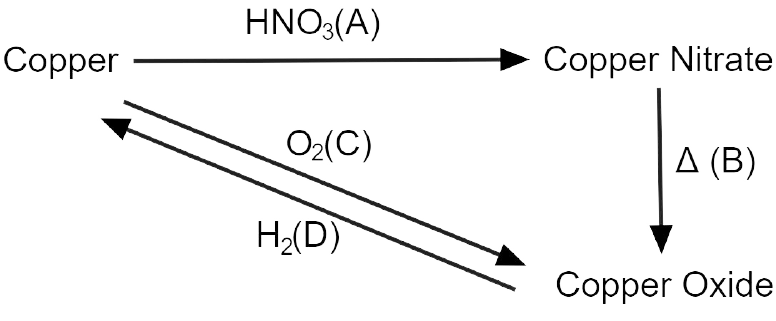
Explanation:
3 Cu + 8 HNO3 \[\to \] 3 Cu(NO3)2 + 4 H2O + 2 NO
2 Cu(NO3)2 \[\to \] 2 Cu + 4 NO2 + O2 (Decomposition)
2 Cu + O2 \[\to \] 2 CuO (Oxidation)
CuO + H2 \[\to \] Cu + H2O (Reduction)
11: Correct the following, if required:
a. HNO3 is a strong reducing agent
Ans: HNO3 is a strong reducing agent - Correct.
NaNO3 gives NO2 and O2 on heating.
Ans: NaNO3 gives NO2 and O2 on heating- Incorrect
NaNO3 gives NaNO2 and O2 on heating- Correct
c. Constant boiling nitric acid contains 80% nitric acid by weight.
Ans: Constant boiling nitric acid contains 80% nitric acid by weight- Incorrect
Constant boiling nitric acid contains 68% nitric acid by weight- Correct
Nitric acid remains colorless even when exposed to light.
Ans: Nitric acid remains colorless even when exposed to light.- Incorrect
Nitric acid turns yellow when exposed to light.- Correct
Exercise Questions
1. Choose the correct answer
a. The nitrate salt which does not give a mixture of NO2 and O2 on heating is:
I. AgNO3
II. KNO3
III. Cu(NO3)2
IV. Zn(NO3)2
Solution: II. KNO3 is the nitrate salt which does not give a mixture of NO2 and O2 on heating.
The alkali metal nitrates such as sodium or potassium nitrates when heated melts into colourless liquids which decompose on heating to give oxygen gas. The colorless liquid burns into flame due to the formation of oxygen gas when the glowing splinter is brought near.
2 KNO3 \[\to \] 2 KNO2 + O2
Colourless Crystalline
(b) The chemical used in the brown ring test is:
I. CuSO4
II. FeSO4
III. Fe2(SO4)3
IV. ZnSO4
Ans: II. The chemical used in the brown ring test is FeSO4.
Explanation: The brown ring is formed at the junction of ferrous sulphate and sulphuric acid as the conc. sulphuric acid being heavier settles down and the ferrous sulphate layer remains above it resulting in the formation of a brown ring at the junction as the reaction given below:
6 FeSO4 + 3 H2SO4 + 2 HNO3 \[\to \] 3 Fe2(SO4)3 + 4 H2SO4 + NO
FeSO4 + NO \[\to \] FeSO4 . NO
(Nitroso Ferrous sulfate, a brown complex)
(c) Lead nitrate decomposes on heating to give:
I. NO
II. N2O
III. NO2
IV. N2O5
Ans: II. Lead nitrate decomposes on heating to give NO2.
Lead nitrate decomposes on heating to give lead oxide, nitrogen dioxide and oxygen.
2 Pb(NO3)2 \[\to \] 2 PbO + 4 NO2 + O2
Yellow solid
2. Name
a) a nitrate of metal which on heating does not give nitrogen dioxide.
Ans: A nitrate of metal that on heating does not give nitrogen dioxide is sodium nitrate.
b) a nitrate which on heating leaves no residue behind.
Ans: A nitrate that on heating leaves no residue behind is ammonium nitrate.
c) a metal nitrate which on heating is changed into metal oxide.
Ans: A metal nitrate that on heating is changed into metal oxide is calcium nitrate.
d) a metal nitrate which on heating is changed into metal.
Ans: A metal nitrate that on heating is changed into metal oxide is mercury nitrate.
e) a solution that absorbs nitric oxide.
Ans: A solution that absorbs nitric oxide is a ferrous sulfate solution.
f) the oxide of nitrogen which turns brown on exposure to air. How is it prepared?
Ans: Nitric oxide is the oxide of nitrogen that turns brown on exposure to air.
2 NO + O2 \[\to \] NO2
Nitric Oxide Nitrogen Dioxide (brown gas)
Nitric oxide can be prepared during catalytic oxidation by platinum at 800C of ammonia as shown below:
4 NH3 + 5 O2 \[\to \] 4 NO + 6 H2O + Heat
Explanation:
Alkali metals such as sodium and potassium nitrate melts into colourless liquid on heating and decomposes to give oxygen.
2 NaNO3 \[\to \] 2 NaNO2 + O2
2 KNO3 \[\to \] 2 KNO2 + O2
When a glowing splinter is brought near the molten liquid, it burst into flames as oxygen gas is evolved.
All other nitrates except those of silver and mercury decompose to give their oxides, nitrogen dioxide and oxygen.
2 Ca(NO3)2 \[\to \] 2 CaO + 4 NO2 + O2
Brown gas
2 Zn(NO3)2 \[\to \] 2 ZnO + 4 NO2 + O2
2 Pb(NO3)2 \[\to \] 2 PbO + 4 NO2 + O2
2 Cu(NO3)2 \[\to \] 2 CuO + 4 NO2 + O2
Silver and mercury nitrates decompose to give their respective metals nitrogen dioxide and oxygen.
2 AgNO3 \[\to \] 2 Ag + 2 NO2 + O2
2 Hg(NO3)2 \[\to \] Hg + 2 NO2 + O2
Ammonium nitrate decomposes explosively to give no residue as N2O gas is evolved and water as steam evaporated leaving behind no residue.
2 NH4NO3 \[\to \] N2O(g) + 2 H2O
Nitrous Oxide (Laughing gas)
Ferrous sulphate solution absorbs nitric oxide to form nitroso ferrous sulphate, a brown complex.
2 FeSO4 + H2O + NO \[\to \] [Fe (H2O)NO]SO4
Nitric Oxide Brown Complex
Nitric oxide turns brown on exposure to air due to formation of nitrogen dioxide (brown gas). Nitric oxide can be prepared by catalytic oxidation by platinum at 800C of ammonia.
3. Mention three important uses of nitric acid. Give the property of nitric acid involved in the use.
Ans:
S.No. | Uses | Property of Nitric Acid |
1. | To etch designs on copper and brasswares | Acts as solvent for a large number of metals except noble metals |
2. | Purification of gold | Dissolves impurities such as Ag, Cu, Zn etc. present in gold |
3. | Preparation of Aqua regia | Highly corrosive, dissolves noble metals |
4. (a) Explain with the help of a balanced equation, the brown ring test for nitric acid.
Ans: In the brown ring test for nitric acid, the brown ring is formed at the junction of ferrous sulphate and sulphuric acid as the conc. sulphuric acid being heavier settles down and the ferrous sulphate layer remains above it resulting in the formation of a brown ring at the junction due to the formation of brown complex compound as the reaction given below:
6 FeSO4 + 3 H2SO4 + 2 HNO3 \[\to \] 3Fe2(SO4)3 + 4 H2SO4 + NO
FeSO4 + NO \[\to \] FeSO4. NO
(Nitroso Ferrous sulphate, a brown compound)
b. Why is a freshly prepared ferrous sulphate solution used for testing the nitrate radical in the brown ring test?
Ans: A freshly prepared ferrous sulphate solution is used for testing the nitrate radical in the brown ring test because on exposure to the atmosphere, it is oxidised to ferric sulphate due to which it will not give the brown ring test.
5. From the following list of substances, choose one substance in each case that matches the description given below:
Ammonium nitrate, calcium hydrogen carbonate, copper carbonate, lead nitrate, potassium nitrate, sodium carbonate, sodium hydrogen carbonate, zinc carbonate.
A substance that gives off only oxygen when heated.
Ans: Potassium nitrate gives off only oxygen when heated.
Alkali metals such as sodium and potassium nitrate melts into colourless liquid on heating and decompose to give oxygen.
2 NaNO3 \[\to \] 2 NaNO2 + O2
2 KNO3 \[\to \] 2 KNO2 + O2
When a glowing splinter is brought near the molten liquid, it burst into flames as oxygen gas is evolved.
b. A substance that on heating decomposes into dinitrogen oxide (nitrous oxide) and steam.
Ans: Ammonium nitrate on heating decomposes into dinitrogen oxide (nitrous oxide) and steam.
Ammonium nitrate decomposes explosively to give no residue as
2 NH4NO3 \[\to \] N2O(g) + 2 H2O
Nitrous Oxide (Laughing gas)
c. A substance that gives off oxygen and nitrogen dioxide when heated.
Ans: Lead nitrate off oxygen and nitrogen dioxide when heated.
Explanation:
All other nitrates except those of silver and mercury decompose to give their oxides, nitrogen dioxide and oxygen.
2 Ca(NO3)2 \[\to \] 2 CaO + 4 NO2 + O2
Brown gas
2 Zn(NO3)2 \[\to \] 2 ZnO + 4 NO2 + O2
2 Pb(NO3)2 \[\to \] 2 PbO + 4 NO2 + O2
2 Cu(NO3)2 \[\to \] 2 CuO + 4 NO2 + O2
d. A substance which on heating leaves yellow residue
Ans: Zinc carbonate on heating leaves yellow residue.
Explanation:
Zinc carbonate on heating form zinc oxide and carbon dioxide gas is evolved leaving behind the yellow ZnO.
ZnCO3 \[\to \] ZnO + CO2 + O2
Yellow solid when hot,
white when cold
6. The action of heat on the blue crystalline solid X, gives a reddish-brown gas Y, a gas that re-lights a glowing splint and leaves a black residue. When gas Z, which has a rotten egg smell, is passed through a solution of X. a black ppt. is formed.
a. Identify X, Y, and Z.
Ans: X is copper nitrate [Cu(NO3)2], Y is nitrogen dioxide [NO2] and Z is hydrogen sulfide [H2S].
b. Write an equation for the action of heat on X.
Ans: When copper nitrate [Cu(NO3)2] is heated, a brown color gas of nitrogen dioxide NO2 with oxygen gas is evolved leaving behind a black residue of copper oxide, CuO. As oxygen gas is evolved, glowing splints will re-lights.
2 Cu(NO3)2 \[\to \] 2 CuO + 4 NO2 + O2
Reddish-brown
c. Write equation between the solution of X and gas Z.
Ans: Cu(NO3)2 + H2S \[\to \] CuS + 2 HNO3
Explanation:
Salt of copper and their solution are blue in colour. Copper nitrate [Cu(NO3)2] is a blue crystalline solid X, when heated, gives reddish-brown NO2 gas, oxygen gas which re-lights a glowing splint and leaves a black residue of CuO.
2 Cu(NO3)2 \[\to \] 2 CuO + 4 NO2 + O2
Reddish-brown
When a H2S gas, which has a rotten egg smell, is passed through a solution of Cu(NO3)2, a black ppt. of CuS is formed.
2 Cu(NO3)2 + H2S \[\to \] 2 CuS + 2 HNO3
Black ppt.
7. X. Y and Z are three crystalline solids that are soluble in water and have a common anion. To help you to identify X. Y and Z, you are provided with the following experimental observations. Copy and complete the corresponding inferences in (a) to (e).
a. A reddish-brown gas is obtained when X, Y and Z are separately warmed with concentrated sulphuric acid and copper turning added to the mixture.
INFERENCE I: The common anion is the _____________ ion.
Ans: The common anion is the nitrate ion.
As the reddish-brown gas is obtained when X, Y, and Z are separately warmed with concentrated sulphuric acid when these have anion as nitrate which evolve into brown NO2 gas that intensifies when copper turning added to this acidified mixture.
Metallic nitrates \[\to \] Metallic Oxides + NO2 + O2
b. When X is heated, it melts and gives off only one gas which re-lights a glowing splint.
Inference 2: The cation in X is either __________ or ___________.
Ans: The cation in X is either sodium or potassium.
Sodium and potassium nitrates or alkali nitrates melts into colorless liquid on heating and decomposes to give oxygen.
2 NaNO3 \[\to \] 2 NaNO2 + O2
Slightly delinquent
KNO3 \[\to \] KNO2 + O2
Colorless Crystalline
When the glowing splinter is brought near, the colorless liquid, it re-lights due to the evolution of oxygen gas.
c. The action of heat on Y produces a reddish-brown gas and a yellow residue which fuses with the glass of the test tube.
Inference 3: The metal ion present in Y is the ___________ ion.
Ans: The metal ion present in Y is the lead ion.
Lead nitrate is Y which is a colorless crystalline solid when heated from a yellow solid of lead oxide which fuses with glass and reddish-brown nitrogen dioxide gas is evolved along with oxygen.
2 Pb(NO3)2 \[\to \] 2 PbO + 4 NO2 + O2
Colorless Yellow solid
Crystals fuses with glass
d. When Z is heated, it leaves no residue. Warming Z with sodium hydroxide solution liberates a gas which turns moist red litmus paper blue.
Inference 4: Z contains the __________ ion.
Ans: Z contains the ammonium cation.
Ammonium nitrate decomposes explosively to give no residue as N2O gas is evolved and water as steam evaporated leaving behind no residue.
2 NH4NO3 \[\to \] N2O(g) + 2 H2O
Nitrous Oxide (Laughing gas)
When ammonium nitrate gives ammonia gas which is basic in nature and moist red litmus paper blue.
2 NH4NO3 + NaOH \[\to \] NaNO3 + NH3 + H2O
e. Write the equations for the following reactions.
(i) X and concentrated sulphuric acid (below 210° C). (One equation only for either of the cations given in INFERENCE 2).
Ans: X can be sodium and potassium nitrates and their reactions with concentrated sulphuric acid (below 210°C) form. When sodium and potassium nitrates react with concentrated sulphuric acid form nitric acid.
2 NaNO3 + H2SO4 \[\to \] Na2SO4 + 2 HNO3
KNO3 + H2SO4 \[\to \] KHSO4 + HNO3
(ii) Action of heat on Y.
Ans: Y, which is lead nitrate decomposes on heat and form a yellow solid of lead oxide which fuses with glass and reddish-brown nitrogen dioxide gas is evolved along with oxygen.
2 Pb(NO3)2 \[\to \] 2 PbO + 4 NO2 + O2
Colorless Yellow solid
Crystals fuses with glass
(iii) Concentrated nitric acid is added to copper turnings kept in a beaker.
Ans: When concentrated nitric acid is added to copper turnings kept in a beaker, dense reddish-brown fumes of nitrogen dioxide gas are evolve.
Cu + 4 HNO3 \[\to \] Cu(NO3)2 + 2 H2O + 2 NO2
Reddish brown
8.(a) Dilute nitric acid is generally considered a typical acid except for its reaction with metals. In what way is dilute nitric acid different from other acids when it reacts with metals?
Ans: Generally, acids react with metals to form salts and hydrogen gas but not the same case with nitric acid. Dilute nitric acid is considered a typical acid except for its reaction with metals since it does not liberate hydrogen. It is a powerful oxidising agent and the nascent oxygen formed oxidises the hydrogen to water.
All metals (except Mn and Mg) react with dil. nitric acid to evolve nitrate salt of corresponding metal, water and nitric oxide.
3 Cu + 8 HNO3 \[\to \] 3 Cu(NO3)2 + 4 H2O + 2 NO
3 Zn + 8 HNO3 \[\to \] 3 Zn(NO3)2 + 4 H2O + 2 NO
3 Fe + 8 HNO3 \[\to \] 3 Fe(NO3)2 + 4 H2O + 2 NO
b. Write the equation for the reaction of dilute nitric acid and conc. nitric acid with copper.
Ans: The equation for the reaction of dilute nitric acid and conc. nitric acid with copper is:
Reaction with dil. HNO3:
3 Cu + 8 HNO3 \[\to \] 3 Cu(NO3)2 + 4 H2O + 2 NO
Reaction with conc.HNO3:
Cu + 4 HNO3 \[\to \] 3 Cu(NO3)2 + 2 H2O + 2 NO2
9. Explain why
a. Only all-glass apparatus should be used for the preparation of nitric acid by heating concentrated sulphuric acid and potassium nitrate.
Ans: Only all-glass apparatus should be used for the preparation of nitric acid by heating concentrated sulphuric acid and potassium nitrate because fumes of nitric acid formed during the reaction are acidic and highly corrosive and react with cocks, rubber etc. if used as stopper.
b. Nitric acid is kept in a reagent bottle for a long time.
Ans: Nitric acid is kept in a reagent bottle for a long time because pure nitric acid is unstable to sunlight and heat and to avoid its decomposition it is kept in reagent bottle.
4 HNO3 \[\to \] O2 + 2 H2O + 2 NO2
Due to the formation of brown NO2 gas, nitric acid turns yellowish in color because of the dissolution of NO2 gas in HNO3.
10. The figure given below illustrates the apparatus used in the laboratory preparation of nitric acid.
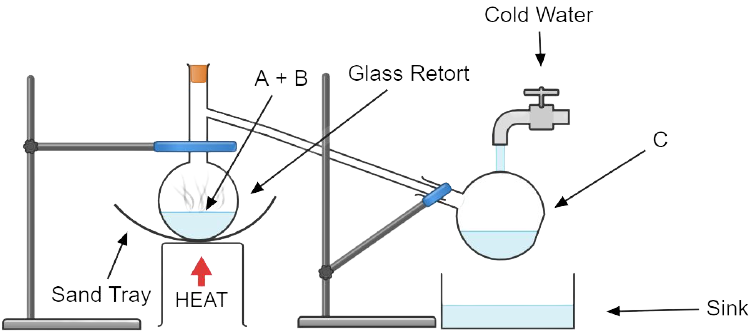
a. Name A (a liquid), B (a solid) and C (a liquid). (Do not give the formulae).
Ans: A(a liquid) is concentrated sulphuric acid, B (a solid) is sodium nitrate or potassium nitrate and C (a liquid) is nitric acid.
b. Write an equation to show how nitric acid undergoes decomposition.
Ans: Nitric acid decomposes to liberate nitrogen dioxide along with oxygen and form water.
4 HNO3 \[\to \] O2 + 2 H2O + 2 NO2
c. Write the equation for the reaction in which copper is oxidized by concentrated nitric acid.
Ans: Copper metal is oxidized by concentrated nitric acid to copper nitrate.
Cu + 4 HNO3 \[\to \] Cu(NO3)2 + 2 H2O + 2 NO2
11.
a. A dilute acid B does not normally give hydrogen when reacted with metals but does give a gas when reacts with copper. Identify B. Write equation with copper.
Ans: Acid B is nitric acid as it oxidises the hydrogen formed when reacted with metals to form water and hence does not normally give hydrogen gas. Nitric acid reacts with copper to evolve nitrogen dioxide gas.
Cu + 4 HNO3 \[\to \] 3 Cu(NO3)2 + 2 H2O + 2 NO2
b. Complete the table:
Name of Process | Inputs | Equation | Output |
Ammonia + Air | Nitric Acid |
Ans:
Name of Process | Inputs | Equation | Output |
Ostwald Process | Ammonia + Air | 4 NH3 + 5O2 ⟶ 4 NO + 6H2O + Heat 2 NO + O2 ⟶ 2 NO2 4 NO2 + 2 H2O + O2 ⟶ 4 HNO3 | Nitric Acid |
c. What is the property of nitric acid which allows it to react with copper?
Ans: Oxidising property of nitric acid allows it to react with copper.
d. State one observation:
i) Concentrated nitric acid is reacted with sulphur.
Ans: When concentrated nitric acid is reacted with sulphur, dense brown fumes of nitrogen dioxide are observed.
S + 6 HNO3 \[\to \] H2SO4 + H2O + 6 NO2
ii) Lead nitrate is heated strongly in a test tube.
Ans: Lead nitrate decomposes on heating strongly in a test and brown gas of nitrogen dioxide is observed.
2 Pb(NO3)2 \[\to \] 2 PbO + 4 NO2 + O2
Yellow solid
2012:
a. Name - the gas produced when copper reacts with conc. nitric acid.
Ans: Nitrogen gas is evolved when copper reacts with conc. nitric acid.
Cu + 4 HNO3 \[\to \] Cu(NO3)2 + 2 H2O + 2 NO2
Reddish brown
b. State observation - zinc nitrate crystals are strongly heated.
Ans: When zinc nitrate crystals are strongly heated, brown fumes of nitrogen dioxide gas is observed leaving behind the yellow residue of zinc oxide which turns white when cooled.
2 Zn(NO3)2 \[\to \] 2 ZnO + 4 NO2 + O2
Yellow(when hot),
white(when cold)
c. Correct the statement:
Magnesium reacts with nitric acid to liberate hydrogen gas.
Ans: Magnesium reacts with nitric acid to liberate hydrogen gas.- Incorrect
Magnesium reacts with dilute nitric acid to liberate hydrogen gas. - Correct.
Very dilute (about 1%) acid reacts with magnesium (and manganese) at room temperature to give its nitrates and hydrogen gas. The oxidizing action of the acid is much reduced due to the dilution of the acid.
Mg + 2 HNO3 \[\to \] Mg(NO3)2 + H2
(Very Dilute)
d. Iron is rendered passive with fuming HNO3.
Ans: Iron forms a thin layer of insoluble iron oxide with fuming HNO3 which makes it passive (or inert).
e. Give the balanced equation for dilute nitric acid and copper carbonate.
Ans: CuCO3 + 2 HNO3 \[\to \] Cu(NO3)2 + H2O + CO2
2013:
a. Identify the gas evolved when.
i) Sulphur is treated with conc. nitric acid.
Ans: Nitrogen dioxide gas is evolved when sulphur is treated with conc. nitric acid.
S + 6 HNO3 \[\to \] H2SO4 + 2H2O + 6 NO2
ii) A few crystals of KNO3 are heated in a hard glass test tube.
Ans: Oxygen gas is evolved when a few crystals of KNO3 are heated in a hard glass test tube.
2 KNO3 ⟶ 2 KNO2 + O2
b. State two relevant observations for lead nitrate crystals heated in a hard glass test tube.
Ans: The two relevant observations for lead nitrate crystals heated in a hard glass test tube are:
i) Brown fumes of nitrogen dioxide gas evolved.
ii) Yellow solid PbO which fuses with glass is formed as a residue.
2 Pb(NO3)2 \[\to \] 2 PbO + 4 NO2 + O2
Yellow solid
c. Give a balanced equation for oxidation of carbon with conc. HNO3.
Ans: The balanced equation for oxidation of carbon with conc. HNO3 is:
C + 4 HNO3 \[\to \] CO2 + 2 H2O + 4 NO2
conc.
2014:
a. Fill In the blank
Cold dil. nitric acid reacts with copper to form _________ (hydrogen, nitrogen dioxide. nitric oxide).
Ans: Cold dil. nitric acid reacts with copper to form nitric oxide.
3 Cu + 8 HNO3 \[\to \] 3 Cu(NO3)2 + 4 H2O + 4 NO
Nitric oxide
b. Give balanced equations for the following:
I. Laboratory preparation of nitric acid.
Ans: KNO3 + H2SO4 \[\to \] 2 KHSO4 + HNO3
Conc.
NaNO3 + H2SO4 \[\to \] 2 NaHSO4 + HNO3
Conc.
Nitrate acid is prepared in the laboratory by heating the sodium or potassium nitrate salt above 200C with concentrated sulphuric acid.
II. The action of heat on a mixture of copper and nitric acid.
Ans: Cu + 4 HNO3 \[\to \] Cu(NO3)2 + 2 H2O + 2 NO2
Reddish brown
2015
a. Identify the acid
I. Which is used for the preparation of non-volatile acid.
Ans: Nitric acid is used for the preparation of non-volatile acid
S + 6 HNO3 \[\to \] H2SO4 + 2H2O + 6 NO2
Non-volatile Acid
II. The acid is prepared by catalytic oxidation of ammonia.
Ans: Nitric acid is the acid which is prepared by catalytic oxidation of ammonia.
4 NH3 + 5 O2 \[\to \] 4 NO + 6 H2O + Heat
2 NO + O2 \[\to \] 2 NO2
4 NO2 + 2 H2O + O2 \[\to \] 4 HNO3
b. State one appropriate observation; when crystals of copper nitrate are heated in a test tube.
Ans: When crystals of copper nitrate are heated in a test tube, the evolution of brown gas of nitrogen dioxide is observed.
2 Cu(NO3)2 \[\to \] 2 CuO + 4 NO2 + O2
Reddish-brown
c. Explain the following:
I. Dil. HNO3 is generally considered a typical acid but not so in the reaction with metals.
Ans: Generally acids react with the metal to evolve hydrogen gas but not so for the reaction of dil. HNO3 with metals because nitric acid acts as an oxidizing agent and oxidizes the evolved hydrogen gas to water. Hence, dil. HNO3 is not considered a typical acid.
II. When it is left standing in a glass bottle concentrated nitric acid appears yellow.
Ans: Concentrated nitric acid is unstable to sunlight and heat and so decomposes when it is left standing in a glass bottle.
4 HNO3 \[\to \] O2 + 2 H2O + 2 NO2
Reddish brown
The formed brown nitrogen dioxide gas dissolves in the nitric acid due to which it appears yellow in color.
III. In the laboratory preparation of nitric acid, an all-glass apparatus is used.
Ans: Nitric acid is a highly corrosive acid, the fumes of nitric acid can corrode the cork, and rubber is used as a stopper in the laboratory preparation of nitric acid so as to avoid corrosion of the apparatus an all-glass apparatus is used.
Introduction to Nitric Acid
Many industrial and commercial products are manufactured using nitric acid, which is one of the most important compounds. Thus, it is very important for science students to become familiar with nitric acid early on and obtain a thorough understanding. This chapter basically encapsulates the essence of this topic because students learn about the laboratory procedure of preparing nitric acid from potassium nitrate or sodium nitrate in this chapter. In addition, Chemistry Class 10 Solutions for Chapter 10 directs students to examine the experiments from the viewpoint of reactants, equations, settings, products, conditions, diagrams, precautions, and collections.
The synthesis of nitric acid was first recorded around 1500. Nitric acid is also known as aqua fortis and spirits of nitre. Alchemical work in the year 800 AD by Jabir ibn Hayyan a.k.a. Gerber.
HNO3 is the formula for the reaction
with a molecular mass of 63.01 grams per mole
densities of 1.51 grams/cubic centimetre
with boiling points of 83°C
and melting points of -42°C
The acid has a pungent odour and a suffocating smell. It is highly toxic to inhale and highly corrosive to metals and tissues.
As part of acid rain, nitric acid is emitted. Water vapour in the air and nitrogen oxides emitted from automobile engines combine to form nitric acid. When this acid rain falls to earth, it causes harm to plants and animals.
It is prepared by oxidizing nitrogen dioxide (NO2) in the presence of a platinum catalyst at high temperatures (Ostwald process) to form nitric oxide. Commercially, nitric acid is produced by oxidizing anhydrous ammonia to nitric oxide.
Students preparing for the board exams can find maximum information in Solutions for Class 10 Study of Compounds Nitric Acid. The answers to the questions are step-by-step and simple to understand. Using them, students can very quickly understand the concepts behind the solutions. Students will not only benefit by being more prepared to answer all sorts of questions in examinations, but they will also have a better chance of retaining the information. During the chapter on Nitric Acid Solutions Class 10, students are encouraged to answer questions in a correct manner so that they can achieve maximum marks.
Preparation Tips for Class 10 Chemistry Exam
Learn the topics covered in your Chemistry textbooks like acids, bases, salts, and carbon and its compounds, and analyze how these topics apply to real-life scenarios, as science can be found in everything.
Solve Sample Papers
Exam simulation helps you test your skills in a real exam room. By solving sample papers, you get the chance to create the atmosphere of an exam room. You will be able to think fast in stressful situations when you are using this strategy. This is great if you want to practice in preparation for exams.
Use Mnemonics
In your Chemistry syllabus, there are many topics that require more memorization rather than solving, which is a difficult challenge for all students. Mnemonics are useful when dealing with such topics as they are creative tools that aid in the retention of information.
A Study from the Prescribed Textbooks
ICSE Board question papers are based strictly on the syllabus that the board sets, so it is an absolute must that you examine your prescribed textbooks rather than relying on too many other sources.
Always Balance the Equations
Regardless of what the question requires, you should write chemically balanced equations. Make a list of balanced equations and use mnemonics to remember them all if you are having difficulty memorizing them.
FAQs on Study of Compounds-Nitric Acid Solutions for Class 10 Chemistry
1. Describe three important applications of Nitric acid. Include the property of the acid that is used in these applications.
Nitric acid is used for and has the following properties:
The two acidic testing solutions are used to purify gold alloys. They act in a reaction by dissolving all of the metals in the alloys. Copper and silver dissolve in nitric acid when they are oxidized, as described in the following equation. Copper and silver within the gold alloy speed up the process of dissolving.
A property of nitric acid is that it dissolves impurities such as copper, silver, zinc, etc.Engraves designs on copper using this tool.
The solvent capacity of nitric acid is large, making it useful for many metals.Aqueous Regia can be prepared from it.The property of this substance is that it dissolves noble metals.
2. According to Class 10 Chemistry, what are the methods of preparing Nitric acid?
Nitric acid can be prepared using the following methods:
Sodium nitrate or potassium nitrate are heated together with concentrated sulfuric acid to form nitric acid in the laboratory.
KNO3 + H2SO4 → KHSO4 + HNO3
potassium nitrate sulphuric acid Nitric acid
NaNO3 + H2SO4 → NaHSO4 + HNO3
Sodium nitrate sulphuric acid Nitric acid
Ostwald's process is used commercially to prepare it. In this process, ammonia is oxidized by catalysis and converted into nitric oxide.
Pt/Rh gauze catalyst
4NHO3(g) + SO2(g) → 4NO(g) + 6H2O(g)
potassium nitrate sulphuric acid 1155 K, 9 bar Nitric oxide
The nitric oxide then becomes nitrogen dioxide, which is oxidized to form nitric acid.
2NO(g) + O2(g) → 2NO2(g)
Nitric oxide Oxygen Nitrogen dioxide
3NO2(g) + H2O(g) → 2HNO3(aq) + NO(g)
Nitrogen dioxide Water Nitric acid
Nitric acid contains about 98% concentration, also known as fuming nitric acid.
3. According to Solutions for Compounds-Nitric Acid, how are these compounds relevant?
Nitric acid has the following properties:
The colourless liquid boils at 84.10°C and freezes to a white solid at -41.550°C; it is a gas at room temperature.
The production of brown nitrogen dioxide gas results from the photochemical dissociation of nitric acid. Yellow colour is produced when nitrogen dioxide dissolves in colourless nitric acid.
Sunlight
4HNO3 → 4NO2 + 2H2O
Nitric acid Nitrogen dioxide
Nitric acid is ionized to give off hydronium ions and nitrate ions when it is dissolved in water.
(aq)HNO3 + (l)H2O + → (aq)H3O-(l)
Nitric acid Hydronium ions Nitrate ions
As a result, it's a strong acid. nitric acid reacts powerfully with oxygen when it is hot and concentrated. Several metals, such as copper and zinc, can't dissolve in hydrochloric acid. Nitric acid, on the other hand, dissolves them. When concentrated nitric acid reacts with copper and zinc, nitrogen dioxide is produced.
In order to solve Cu + 4HNO3, multiply it by Cu(NO3)2 + 2NO2 + 2H2O
Copper Nitric acid(Conc.) Copper nitrate Nitrogen dioxide
The following formula is: Zn(NO3)2 + 2NO2 + 2H2O
The nitrogen dioxide in zinc nitrate and zinc oxide is composed of zinc nitrate and zinc oxide.
4. What are the benefits of studying Chemistry in Class 10?
It is no surprise that Chemistry is such a fascinating field. As a natural science that pertains to the entire existence of all living beings, Chemistry is fundamental to the way we live and touches almost every aspect of our existence. For everything from food to clothing to shelter to health, energy, and clean air, water, and soil, Chemistry is necessary. Various aspects of chemical technology improve the quality of our lives in many ways, such as by creating products that solve health, material, and energy-related problems. We can prepare ourselves for real-world situations by studying Chemistry.
Chemical Science connects Physics, Mathematics, Biology, Medicine, Earth Science, and Environmental Science, which is why it is often called the central science. Consequently, chemical knowledge and understanding of chemical processes can help us gain insight into a wide range of physical and biological phenomena. For more details, you can also click on Vedantu.
































