
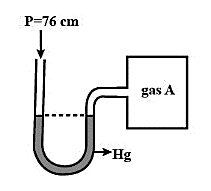
An open-ended mercury manometer is used to measure the pressure exerted by a trapped gas as shown in the figure. Initially, manometer shows no difference in mercury level in both columns as shown in the diagram.
After sparking, ‘A’ dissociates according to the following reaction:
$A(g)\to B(g)+3C(g)$
If the pressure of gas ‘A’ decreases to $0.9atm$. Then:
(assume temperature to be constant and is 300K)
This question has multiple correct options.
(A) total pressure increased to $1.3atm$
(B) total pressure increased by $0.3atm$
(C) total pressure increased by $22.3\,cm\,of\,Hg$
(D) the difference in mercury level is $228\,mm$
Answer
232.8k+ views
Hint: The manometer is a device which measures the pressure exerted by the trapped gas in the container, that is seen through the rise in the mercury level in the two vertical columns. As the pressure increases, it causes the mercury level to rise.
Complete step by step solution:
It is given that in the system, the atmospheric pressure, ${{P}_{i}}=76\,cm\,=1\,atm$. Since initially no pressure is exerted by the gas A trapped in the container and the level of mercury is equal in both the columns. The pressure of gas A is also equal to the atmospheric pressure and the pressure from gas B and C is zero initially.
But as given, on sparking the pressure of gas A decreases to $0.9atm$. Then, the difference in pressure of gas A will be $(initial\,pressure-final\,pressure)=1-0.9=0.1\,atm$.
Let the decrease in the pressure be X.
As the reaction follows: $A(g)\to B(g)+3C(g)$
The pressure of gas A decreases to $1-X=0.9\, atm$.
The pressure of gas B is $X=0.1\,atm$ and pressure of gas C is $3X=3\times 0.1=0.3\,atm$ .
Then, the total pressure exerted within the system, ${{P}_{t}}=(1-X)+X+3X=1.3atm$.
So, we get the increase in the pressure of the system due to gas A undergoing sparking, causing the mercury level to rise in the column.
Thus, total pressure is increased by$\Delta P={{P}_{t}}-{{P}_{i}}=1.3-1=0.3\,atm$
$=0.3\times 76\,cm\,for\,Hg$
$=0.3\times 760\,mm\,=228\,mm\,for\,Hg\,$
This increase in the total pressure of the system, $\Delta P$ is proportional to the difference in mercury level.
Therefore, in the manometer it is seen that there is option (A)- total pressure increased to $1.3atm$, option (B)- total pressure increased by $0.3atm$ and option (D)- the difference in mercury level is $228\,mm$.
Note: The height between the mercury levels in the two columns corresponds to the difference in the pressure exerted by the gas in the container and the atmosphere. Similarly, depending on the pressure of the system the height of mercury in adjacent tubes rises.
Complete step by step solution:
It is given that in the system, the atmospheric pressure, ${{P}_{i}}=76\,cm\,=1\,atm$. Since initially no pressure is exerted by the gas A trapped in the container and the level of mercury is equal in both the columns. The pressure of gas A is also equal to the atmospheric pressure and the pressure from gas B and C is zero initially.
But as given, on sparking the pressure of gas A decreases to $0.9atm$. Then, the difference in pressure of gas A will be $(initial\,pressure-final\,pressure)=1-0.9=0.1\,atm$.
Let the decrease in the pressure be X.
As the reaction follows: $A(g)\to B(g)+3C(g)$
The pressure of gas A decreases to $1-X=0.9\, atm$.
The pressure of gas B is $X=0.1\,atm$ and pressure of gas C is $3X=3\times 0.1=0.3\,atm$ .
Then, the total pressure exerted within the system, ${{P}_{t}}=(1-X)+X+3X=1.3atm$.
So, we get the increase in the pressure of the system due to gas A undergoing sparking, causing the mercury level to rise in the column.
Thus, total pressure is increased by$\Delta P={{P}_{t}}-{{P}_{i}}=1.3-1=0.3\,atm$
$=0.3\times 76\,cm\,for\,Hg$
$=0.3\times 760\,mm\,=228\,mm\,for\,Hg\,$
This increase in the total pressure of the system, $\Delta P$ is proportional to the difference in mercury level.
Therefore, in the manometer it is seen that there is option (A)- total pressure increased to $1.3atm$, option (B)- total pressure increased by $0.3atm$ and option (D)- the difference in mercury level is $228\,mm$.
Note: The height between the mercury levels in the two columns corresponds to the difference in the pressure exerted by the gas in the container and the atmosphere. Similarly, depending on the pressure of the system the height of mercury in adjacent tubes rises.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




