




Difference Between Electron Gain Enthalpy and Electron Affinity (with Table)
The Electron Gain Enthalpy and Electron Affinity topic is crucial for mastering periodic trends and energetics in JEE Main Chemistry. These terms describe the energy changes when a gaseous atom gains an extra electron, influencing reactivity across elements. A firm grip on their definitions, differences, trends, calculations, and exceptions directly uplifts performance in conceptual and numerical questions on atomic structure, the periodic table, and chemical bonding.
Definitions: Electron Gain Enthalpy and Electron Affinity
Electron gain enthalpy (ΔegH) is the amount of energy change when an isolated gaseous atom accepts an electron, forming a negative ion. If energy is released, ΔegH is negative (exothermic); if absorbed, it is positive (endothermic). Electron affinity (EA) is the energy released (taken as positive) when a gaseous atom gains an electron. Both properties highlight how easily atoms form anions, but their sign conventions differ.
Differences Between Electron Gain Enthalpy and Electron Affinity
Although related, electron gain enthalpy and electron affinity differ in sign convention, measurement, and conceptual meaning. These differences are critical for JEE assertion-reason and direct MCQs.
| Parameter | Electron Gain Enthalpy (ΔegH) | Electron Affinity (EA) |
|---|---|---|
| Definition | Energy change when atom gains an electron | Energy released when atom gains an electron |
| Sign Convention | Negative (exothermic), Positive (endothermic) | Always positive when energy is released |
| Measurement | Thermodynamic enthalpy change (ΔegH) | Direct measurement of released energy (EA) |
| Formula Relation | ΔegH = –EA – (5/2)RT | EA = –ΔegH – (5/2)RT |
| Numerical Value | May be negative or positive | Treated as positive when exothermic |
A mnemonic for remembering: Electron affinity is always the amount of energy given out (positive sign), while electron gain enthalpy tracks the system's energy (negative for exothermic processes).
Trends in Periodic Table: Electron Gain Enthalpy & Electron Affinity
Both properties show clear trends across the periodic table that are heavily tested in JEE Main. Across a period (left to right), electron gain enthalpy and electron affinity become more negative/more positive due to increasing nuclear charge and decreasing atomic size, favoring electron acceptance. Down a group, both become less negative due to increased atomic radius and greater electron shielding, making electron addition less favorable.
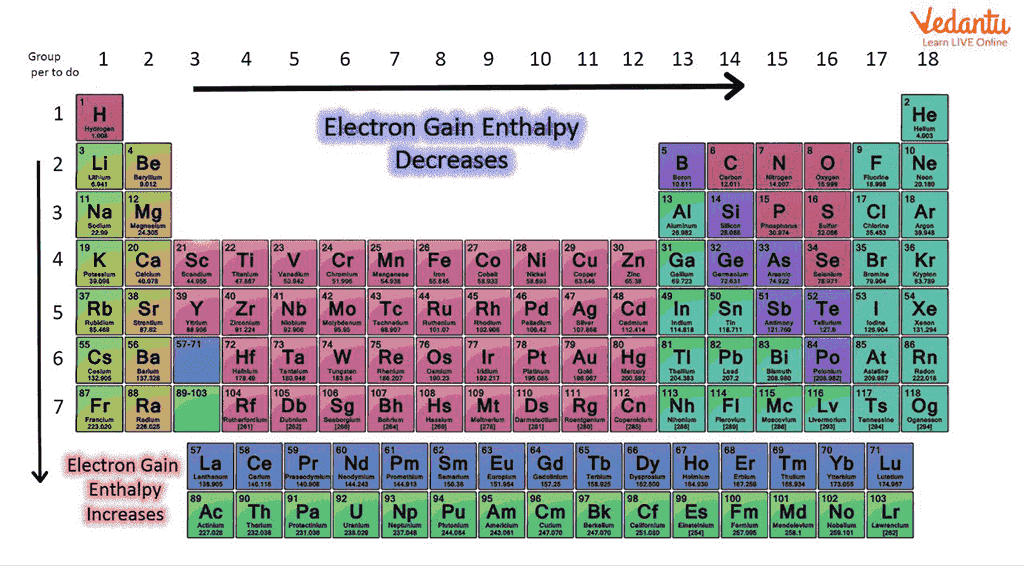
For halogens, with one less electron than noble gas configuration, both values are extreme (Cl: –349 kJ/mol for ΔegH). Noble gases, already stable, have positive electron gain enthalpy and near-zero electron affinity.
- The most negative electron gain enthalpy is for chlorine, not fluorine, due to better size-balance and lower electron repulsion.
- First electron gain is typically exothermic; second is endothermic due to repulsion in already negative ions.
- Practice periodic trends for competitive advantage.
For more on the trend exceptions, especially in halogens, refer to halogen properties and behaviour and p-block pages on Vedantu.
Factors Affecting Electron Gain Enthalpy and Electron Affinity
- Atomic size: Smaller atoms (e.g., O, F) have higher nuclear attraction, but extreme crowding increases repulsion.
- Nuclear charge: Higher nuclear charge (Z) favors electron gain, up to a point.
- Shielding effect: More filled inner shells, lower effective nuclear charge, less negative ΔegH.
- Electronic configuration: Stable configuration (ns2 np6) resists extra electrons.
- Subshell repulsion: Additional electron entering a crowded p-subshell faces more repulsion (F anomaly).
Calculations and Example Problems
A typical JEE question: "Calculate the electron affinity (EA) if the electron gain enthalpy (ΔegH) of oxygen is –141 kJ/mol at 298 K. (R = 8.314 J/mol·K)"
- Formula: ΔegH = –EA – (5/2)RT
- (5/2)RT = (5/2) × 8.314 × 298 = 6191 J = 6.19 kJ
- –141 = –EA – 6.19 → EA = 141 – 6.19 = 134.81 kJ/mol
Remember: Use correct sign conventions—ΔegH may be negative, but EA is always positive for energy released.
Exceptions and Conceptual Notes
- Fluorine has less negative electron gain enthalpy than chlorine due to compact size and high repulsions.
- Noble gases have positive ΔegH and zero/near-zero EA—their shells are full.
- Second electron gain enthalpy for any atom is always positive (endothermic), e.g., O– + e– → O2–.
- Be, Mg, N, P show unexpected low or positive values due to half-filled or fully filled subshell stability.
- Link: Electronegativity vs electron affinity for deeper contrast.
Understanding these exceptions helps in assertion-reason and "select correct statement" type JEE questions. For practice, consult JEE Chemistry papers and mock tests.
Comparative Summary Table: EA vs EN vs Electronegativity
| Property | Electron Gain Enthalpy | Electron Affinity | Electronegativity |
|---|---|---|---|
| Process | Atom gains an electron | Atom gains an electron | Atom attracts shared electrons in bond |
| Sign | Usually negative (exothermic) | Always positive when exothermic | Dimensionless scale |
| Unit | kJ/mol | kJ/mol | Pauling scale |
| Numerical Range | Varies, negative to positive | Positive (for energy released) | 0.7 (Cs) to 4.0 (F) |
| Application | Isolated atoms (gas phase) | Isolated atoms (gas phase) | Bonded atoms in molecules |
Study these distinctions along with atomic structure and bonding for holistic JEE preparation.
Application and Practice: JEE-Standard Question Types
- Arrange elements like O, S, Se, Te by electron gain enthalpy.
- Predict the sign and magnitude of ΔegH for noble gases vs halogens.
- Reason: "Cl has more negative electron gain enthalpy than F because..."
- Numerical: Calculate EA given ΔegH and temperature.
- Connect trends to thermodynamics and bond formation.
For self-testing, access mock tests on classification and periodicity and review topic-wise important questions.
Key Takeaways: Mastering Electron Gain Enthalpy and Electron Affinity
- Sign conventions are vital: negative for electron gain enthalpy, positive for electron affinity.
- Chlorine, not fluorine, has the most negative electron gain enthalpy—size matters!
- Down groups: both EN and EA become less favorable (less negative/more positive).
- Noble gases and stable electronic configurations resist electron addition (positive ΔegH).
- Practice conceptual and numerical Qs for a complete grasp—link with atomic and periodic theory.
To summarize, mastering Electron Gain Enthalpy and Electron Affinity is foundational for JEE-level Chemistry. These properties explain reactivity, trends, and exceptions within the periodic table. Practice with Vedantu's focused study material, take topic-wise p-block element tests, and boost your readiness for challenging assertion-reason and calculation-based JEE Main questions.
FAQs on Electron Gain Enthalpy and Electron Affinity Explained
1. What is the difference between electron gain enthalpy and electron affinity?
Electron gain enthalpy is the energy change when an isolated gaseous atom gains an electron, while electron affinity is the amount of energy actually released in the same process.
Key differences:
- Electron gain enthalpy (ΔegH) may be endothermic or exothermic; electron affinity (EA) is usually considered the magnitude (always positive) of energy released.
- Electron affinity is a measurable quantity; electron gain enthalpy is a calculated thermodynamic value.
- Both concepts relate to the ease with which an atom accepts an extra electron, crucial for periodic table trends and JEE Main chemistry.
2. What do you mean by electron affinity?
Electron affinity is the energy released when a neutral gaseous atom gains an extra electron to form a negative ion.
Main points:
- It is usually expressed in kJ/mol.
- High electron affinity means the atom easily attracts extra electrons.
- Halogens like chlorine and fluorine have the highest electron affinities in the periodic table.
3. How to find the electron gain enthalpy?
The electron gain enthalpy is determined by measuring the energy change when one mole of gaseous atoms gains an electron.
Steps to calculate:
- Write the equation: X(g) + e- → X-(g) + ΔegH
- Measure or use tabulated data for the energy released or absorbed.
- Negative value = energy released (exothermic); positive = energy absorbed (endothermic).
4. Does electron gain enthalpy increase down the group?
Generally, electron gain enthalpy becomes less negative (decreases in magnitude) as you move down a group in the periodic table.
Reasons:
- Atomic size increases, so added electrons feel less attraction from the nucleus.
- Nuclear charge increases but is offset by increased shielding effect.
- This trend has exceptions (e.g., between F and Cl).
5. What is the difference between EA and EN?
EA (Electron Affinity) is the energy released by an atom when it gains an electron, while EN (Electronegativity) is a relative scale indicating how strongly an atom attracts electrons in a chemical bond.
Summary:
- EA is a measurable, quantitative value; EN is a dimensionless, relative property.
- EA refers to isolated atoms; EN applies to atoms in molecules.
- Both properties show similar trends across periods/groups but have different definitions and uses in periodic table analysis.
6. Why does chlorine have higher electron gain enthalpy than fluorine?
Chlorine has a more negative (i.e., higher) electron gain enthalpy than fluorine due to fluorine's small size, which leads to high electron-electron repulsion in its compact 2p orbital.
Key reasons:
- Chlorine's larger atomic size minimizes repulsion for the incoming electron.
- Fluorine's high charge density causes added electron to experience repulsion, reducing energy released.
- This is a notable exception to the general group trend.
7. Can any atom have both positive and negative electron gain enthalpy?
Yes, electron gain enthalpy can be negative (energy released) for most non-metals and positive (energy absorbed) for certain elements like noble gases and alkali metals.
Examples:
- Halogens: Negative (favorable electron addition).
- Noble gases: Positive (unfavorable due to stable configuration).
- Group 2 & 18 elements: Often positive values.
8. Is electron gain enthalpy always exothermic?
No, electron gain enthalpy is not always exothermic.
Details:
- It is exothermic (negative value) for most non-metals because energy is released on electron capture.
- It can be endothermic (positive value) for noble gases or elements with full/half-filled subshells, requiring energy input to add an electron.
9. Are electron affinity and electronegativity the same?
Electron affinity and electronegativity are related but not the same.
Differences:
- Electron affinity measures energy released when an atom gains an electron (isolated atom).
- Electronegativity measures an atom’s tendency to attract shared electrons in a chemical bond.
- EA is a specific quantum value; EN is a dimensionless relative scale.
10. Can non-metals have low electron affinity?
Yes, some non-metals may have relatively low electron affinity values due to electronic configuration or small atomic size causing repulsion.
Examples include:
- Nitrogen (half-filled p orbitals, stability discourages electron gain)
- Oxygen (smaller than expected value due to electron repulsion)
- Exceptions highlight important periodic trends in JEE chemistry.
































