
Explain Davisson -Germer’s experiment with the help of a diagram.
Answer
233.1k+ views
Hint: Here, in the Davisson and Germer experiment, he showed that an electron beam can undergo diffraction when it is passed through the atomic crystals. This in turn shows that the wave nature of electrons as waves can exhibit interference and diffraction patterns.
Complete step by step solution:
Initially, the scientists could only explain the particle nature of electrons but failed to explain the properties related to their wave nature. C.J. Davisson and L.H. Germer conducted an experiment in the year 1927, popularly known as Davisson-Germer’s experiment in order to explain the wave nature of electrons through the electron diffraction process.
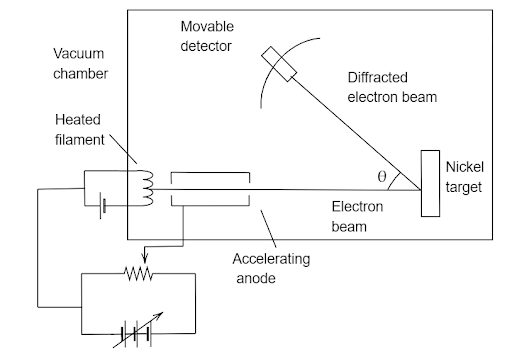
Image: Experimental setup of Davisson Germer experiment
The experimental arrangement and working of the Davisson Germer experiment are discussed below:
An electron gun consisting of a tungsten filament F was coated with barium oxide and heated through a low voltage power supply. While applying the suitable potential difference from a high voltage power supply, the electron gun emits electrons which are again accelerated to a particular velocity. The cylinder was perforated with fine holes along its axis, these emitted electrons were made to pass through it, thus producing a finely collimated beam.
This finely collimated beam produced from the cylinder is again made to fall on the surface of a nickel crystal. Due to this, the electrons will get scattered in various directions and the beam of electrons produced has a certain amount of intensity which is measured by the electron detector and then it is connected to a sensitive galvanometer (to record the current readings), it is then moved on a circular scale.
Then by moving the detector on the circular scale at different positions, that is changing the \[\theta \](angle between the incident beam and the scattered electron beams), the intensity of the scattered electron beam can be measured for different values of angle of scattering.
Let’s see the observations made by Davisson Germer’s experiment. Here we obtain the variation of the intensity (I) of the scattered electrons by changing the angle of scattering, \[\theta \]. The accelerated voltage varied from 44V to 68 V. when we start changing the accelerating potential difference
An accelerating voltage of 54V at a scattering angle \[\theta = {50^0}\], we could see a strong peak in the intensity and this strong peak was the result of constructive interference of the electrons. Using the data of electron diffraction, the wavelength of matter waves was calculated.
Thus, Davisson Germer’s experiment confirms the wave nature of electrons.
Note:Davisson and Germer’s experiments proves the De-Broglie Hypothesis that the electron exists in wave nature as well by this it is possible to understand the structure of particles at the nanoscale using electron diffraction.
Complete step by step solution:
Initially, the scientists could only explain the particle nature of electrons but failed to explain the properties related to their wave nature. C.J. Davisson and L.H. Germer conducted an experiment in the year 1927, popularly known as Davisson-Germer’s experiment in order to explain the wave nature of electrons through the electron diffraction process.

Image: Experimental setup of Davisson Germer experiment
The experimental arrangement and working of the Davisson Germer experiment are discussed below:
An electron gun consisting of a tungsten filament F was coated with barium oxide and heated through a low voltage power supply. While applying the suitable potential difference from a high voltage power supply, the electron gun emits electrons which are again accelerated to a particular velocity. The cylinder was perforated with fine holes along its axis, these emitted electrons were made to pass through it, thus producing a finely collimated beam.
This finely collimated beam produced from the cylinder is again made to fall on the surface of a nickel crystal. Due to this, the electrons will get scattered in various directions and the beam of electrons produced has a certain amount of intensity which is measured by the electron detector and then it is connected to a sensitive galvanometer (to record the current readings), it is then moved on a circular scale.
Then by moving the detector on the circular scale at different positions, that is changing the \[\theta \](angle between the incident beam and the scattered electron beams), the intensity of the scattered electron beam can be measured for different values of angle of scattering.
Let’s see the observations made by Davisson Germer’s experiment. Here we obtain the variation of the intensity (I) of the scattered electrons by changing the angle of scattering, \[\theta \]. The accelerated voltage varied from 44V to 68 V. when we start changing the accelerating potential difference
An accelerating voltage of 54V at a scattering angle \[\theta = {50^0}\], we could see a strong peak in the intensity and this strong peak was the result of constructive interference of the electrons. Using the data of electron diffraction, the wavelength of matter waves was calculated.
Thus, Davisson Germer’s experiment confirms the wave nature of electrons.
Note:Davisson and Germer’s experiments proves the De-Broglie Hypothesis that the electron exists in wave nature as well by this it is possible to understand the structure of particles at the nanoscale using electron diffraction.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Dual Nature of Radiation and Matter Class 12 Physics Chapter 11 CBSE Notes - 2025-26

Understanding the Electric Field of a Uniformly Charged Ring

JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

Derivation of Equation of Trajectory Explained for Students

Understanding Electromagnetic Waves and Their Importance




