
H-O-H bond angle in \[{{H}_{2}}O\] is \[104.5{}^\circ \] and not \[109{}^\circ 28'\] because of
(A) Lone pair-lone pair repulsion
(B) Lone pair-bond pair repulsion
(C) Bond pair-bond pair repulsion
(D) High electronegativity of oxygen
Answer
233.1k+ views
Hint: VSEPR (Valence shell electron repulsion theory) explains the minimal variations we can see from the theoretical bond angles in some of the molecules. As the name suggests it involves repulsion between valence shell orbitals.
Complete step by step solution:
This is the shape of the Water molecule.
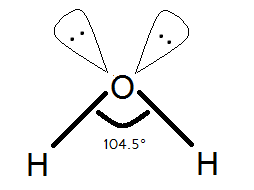
We know and we can see that oxygen has two lone pair on itself. The water molecule has \[s{{p}^{3}}\] hybridization that means it is supposed to have bond angles of \[109{}^\circ 28'\] but there is a special case.
In terms of repulsion, lone pair-lone pair repulsion is higher than any other repulsion.
So, bond pair-bond pair repulsion and lone pair-bond pair repulsion is lower than lone pair- lone pair repulsion.
So, due to lone pair-lone pair repulsion of two lone pairs of the oxygen atom, they both tend to repulse each other and try to stay as far as possible to each other. In that case, the bond angles of \[s{{p}^{3}}\]hybridization do not remain same and have less angle due to that because there is an increase in the angle between two lone pairs and hence there is a decrease in H-O-H angle.
So, that is the reason why Water has H-O-H bond angle of \[104.5{}^\circ \] and not \[109{}^\circ 28'\].
So, the correct answer is (A) Lone pair-lone pair repulsion.
Additional information:
Below are some of the important postulates of VSEPR theory.
-The shape of the molecules depends upon the number of valence cell electron pairs around the central atom.
-Pairs of electrons in valence shell repel each other since electron cloud is negatively charged.
-These electron pairs tend to arrange is space in a way that minimum repulsion is there.
Note: Remember that oxygen atom has two lone pairs in the structure of water molecule. Do not forget to consider the lone pair while predicting the shape or bond angles of a molecule.
Complete step by step solution:
This is the shape of the Water molecule.

We know and we can see that oxygen has two lone pair on itself. The water molecule has \[s{{p}^{3}}\] hybridization that means it is supposed to have bond angles of \[109{}^\circ 28'\] but there is a special case.
In terms of repulsion, lone pair-lone pair repulsion is higher than any other repulsion.
So, bond pair-bond pair repulsion and lone pair-bond pair repulsion is lower than lone pair- lone pair repulsion.
So, due to lone pair-lone pair repulsion of two lone pairs of the oxygen atom, they both tend to repulse each other and try to stay as far as possible to each other. In that case, the bond angles of \[s{{p}^{3}}\]hybridization do not remain same and have less angle due to that because there is an increase in the angle between two lone pairs and hence there is a decrease in H-O-H angle.
So, that is the reason why Water has H-O-H bond angle of \[104.5{}^\circ \] and not \[109{}^\circ 28'\].
So, the correct answer is (A) Lone pair-lone pair repulsion.
Additional information:
Below are some of the important postulates of VSEPR theory.
-The shape of the molecules depends upon the number of valence cell electron pairs around the central atom.
-Pairs of electrons in valence shell repel each other since electron cloud is negatively charged.
-These electron pairs tend to arrange is space in a way that minimum repulsion is there.
Note: Remember that oxygen atom has two lone pairs in the structure of water molecule. Do not forget to consider the lone pair while predicting the shape or bond angles of a molecule.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




