
The correct statement about diborane is:
A. All B–H–B angles are \[{\rm{120^\circ }}.\]
B. Its fragment, \[{\rm{B}}{{\rm{H}}_{\rm{3}}}\] behaves as a Lewis base.
C. Terminal B–H bonds have less p-character when compared to bridging bonds.
D. The two B–H–B bonds are not of the same length.
Answer
233.1k+ views
Hint: Diborane is one of the important hydrides of boron. It is an electron-deficient compound. It has a bridge structure.
Complete Step by Step Solution:
Diborane has a bridge structure in which each boron atom is attached to two hydrogen atoms by regular electron-pair bonds.
These bonds are called terminal bonds.
The bridging H-atoms are in a plane perpendicular to the plane of \[{\rm{B}}{{\rm{H}}_{\rm{2}}}\]fragments which means one lies above and another one lies below the plane.
This prevents the rotation of the two boron atoms.
There are eight bonds in this structure.
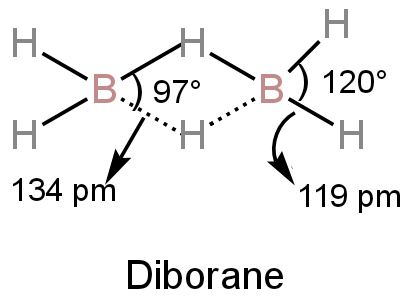
Image: Structure of diborane
A. We can see from the structure of the diborane that the angles of the terminal bonds are \[{\rm{120^\circ }}.\]
The bridge bond's bond angle is \[{\rm{97^\circ }}.\]
So, option A is incorrect.
B. There are eight bonds in this structure and twelve electrons are available for bonding.
This is because the boron has only three valence electrons.
There is one electron contribution from hydrogen.
Two boron atoms contribute six electrons and six electrons are contributed by six hydrogen atoms.
If all the bonds in this molecule will be electron-pair then sixteen electrons are required.
Hence, \[{\rm{B}}{{\rm{H}}_{\rm{3}}}\] is electron-deficient and hence can not act as a Lewis base, but rather acts as a Lewis acid.
Hence, option B is incorrect.
C. The bond angle of terminal bonds is more than the bond angle of bridge bonds.
It means that terminal bond electrons lie farther from the nucleus than the electrons of the bridge bonds.
We know that p-orbitals lie farther from the nucleus than the s-orbitals.
So, the terminal bond electrons lying farther from the nucleus will only result in the p-orbital electrons lying farther from the nucleus.
So, as a result, the p-character decreases.
So, option C is correct.
D.The bond length between boron and hydrogen at the bridges is 134 pm.
The bond length of terminal bonds is 119 pm.
Hence, the four-terminal bonds have the same bond length and the four bridge bonds have the same bond length.
So, option D is incorrect.
So, option C is correct.
Note: While attempting the question, one must have the structure of diborane in mind. The bridge bonds are formed due to the electron deficiency of boron. The terminal B-H bonds are normal two-centre-two-electron bonds while the two bridge bonds are three-centre-two-electron bonds. The bridge bonds are also called banana bonds.
Complete Step by Step Solution:
Diborane has a bridge structure in which each boron atom is attached to two hydrogen atoms by regular electron-pair bonds.
These bonds are called terminal bonds.
The bridging H-atoms are in a plane perpendicular to the plane of \[{\rm{B}}{{\rm{H}}_{\rm{2}}}\]fragments which means one lies above and another one lies below the plane.
This prevents the rotation of the two boron atoms.
There are eight bonds in this structure.

Image: Structure of diborane
A. We can see from the structure of the diborane that the angles of the terminal bonds are \[{\rm{120^\circ }}.\]
The bridge bond's bond angle is \[{\rm{97^\circ }}.\]
So, option A is incorrect.
B. There are eight bonds in this structure and twelve electrons are available for bonding.
This is because the boron has only three valence electrons.
There is one electron contribution from hydrogen.
Two boron atoms contribute six electrons and six electrons are contributed by six hydrogen atoms.
If all the bonds in this molecule will be electron-pair then sixteen electrons are required.
Hence, \[{\rm{B}}{{\rm{H}}_{\rm{3}}}\] is electron-deficient and hence can not act as a Lewis base, but rather acts as a Lewis acid.
Hence, option B is incorrect.
C. The bond angle of terminal bonds is more than the bond angle of bridge bonds.
It means that terminal bond electrons lie farther from the nucleus than the electrons of the bridge bonds.
We know that p-orbitals lie farther from the nucleus than the s-orbitals.
So, the terminal bond electrons lying farther from the nucleus will only result in the p-orbital electrons lying farther from the nucleus.
So, as a result, the p-character decreases.
So, option C is correct.
D.The bond length between boron and hydrogen at the bridges is 134 pm.
The bond length of terminal bonds is 119 pm.
Hence, the four-terminal bonds have the same bond length and the four bridge bonds have the same bond length.
So, option D is incorrect.
So, option C is correct.
Note: While attempting the question, one must have the structure of diborane in mind. The bridge bonds are formed due to the electron deficiency of boron. The terminal B-H bonds are normal two-centre-two-electron bonds while the two bridge bonds are three-centre-two-electron bonds. The bridge bonds are also called banana bonds.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




