
The IUPAC name of the following compound is:
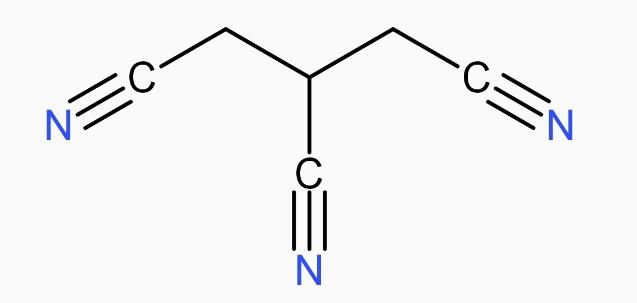
(A) Propane-1,2,3-tricarbonitrile
(B) 3-Cyanopetane-1,5-dinitrile
(C) Pentane-1,3,5-trinitrile
(D) All
Answer
233.1k+ views
Hint: Steps to write the IUPAC name of a compound:
1. Identify the functional group (it can be alcohol, aldehyde, ether etc.).
2. Find the longest carbon chain.
3. Number of carbon atoms present in the longest chain.
4. If there is any branched group, identify their position and name them.
5. Combine the elements' names in a single word.
Complete step by step solution:
* -CN group is named as carbonitrile when they are supposed to yield carboxylic acid on hydrolysis in an aliphatic chain,
* The longest carbon chain here is propane that means 3 carbon, and with each carbon there is one -CN group attached.
* So, the branched CN groups are on the position 1,2,3 and they are three in number. So, we will write ‘tri’ before carbonitrile.
* As we know, the compounds are named in an alphabetical order, thus, propane will be written first.
* Therefore, the IUPAC name of the following compound propane-1,2,3-tricarbonitrile. Its molecular formula is \[{{C}_{6}}{{H}_{5}}{{N}_{3}}\], also the condensed formula is \[CNC{{H}_{2}}CHCNC{{H}_{2}}CN\].
So, the correct option is (a).
Note: Carbon atoms of the terminal groups are not counted in the principal chain. If any one of the terminal groups is not directly attached to the parent chain and forms the part of the side chain, then the longest chain is selected containing two such similar groups at its two ends. The groups present in the side chain are treated as substituents and are indicated by suitable prefixes.
1. Identify the functional group (it can be alcohol, aldehyde, ether etc.).
2. Find the longest carbon chain.
3. Number of carbon atoms present in the longest chain.
4. If there is any branched group, identify their position and name them.
5. Combine the elements' names in a single word.
Complete step by step solution:
* -CN group is named as carbonitrile when they are supposed to yield carboxylic acid on hydrolysis in an aliphatic chain,
* The longest carbon chain here is propane that means 3 carbon, and with each carbon there is one -CN group attached.
* So, the branched CN groups are on the position 1,2,3 and they are three in number. So, we will write ‘tri’ before carbonitrile.
* As we know, the compounds are named in an alphabetical order, thus, propane will be written first.
* Therefore, the IUPAC name of the following compound propane-1,2,3-tricarbonitrile. Its molecular formula is \[{{C}_{6}}{{H}_{5}}{{N}_{3}}\], also the condensed formula is \[CNC{{H}_{2}}CHCNC{{H}_{2}}CN\].
So, the correct option is (a).
Note: Carbon atoms of the terminal groups are not counted in the principal chain. If any one of the terminal groups is not directly attached to the parent chain and forms the part of the side chain, then the longest chain is selected containing two such similar groups at its two ends. The groups present in the side chain are treated as substituents and are indicated by suitable prefixes.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




