
Which of the following is a monomer of Teflon?
(a) Difluoroethane
(b) Trifluoroethane
(c) Tetrafluoroethane
(d) None of these
Answer
233.1k+ views
Hint: Teflon is a polymer which is used widely in our daily lives. It is very popularly used as a non-stick coating in cooking utensils.
Complete step by step answer:
The formula of Teflon is \[{{({{C}_{2}}{{F}_{4}})}_{n}}\]. The structure of Teflon is given below
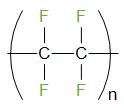
Teflon is also known as PTFE or Polytetrafluoroethene. It is a thermoplastic polymer.
PTFE is manufactured by the addition polymerization of its monomers. It is the reaction which includes free radical polymerization reaction and includes the following steps - initiation, propagation and termination. Therefore, PTFE is an addition polymer.

Tetrafluoroetheneis a monomer of Teflon. The structure of tetrafluoroethene is given below
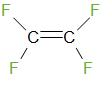
Therefore, the answer is – option (d) – None of these.
Additional Information:
Teflon is the plastic which has the lowest coefficient of friction. As we know, Teflon is very widely used as a non-stick coating for pans and other cookware. Also, being very unreactive Teflon is also very widely used in containers and pipework for reactive chemicals. It is also used as a material to provide resistance to heat & chemical attack. It is also used for making gaskets, pump packing, valves, oil seals, non-lubricated bearings, etc.
Note: The carbons in tetrafluoroethyleneare \[s{{p}^{2}}\] hybridized, where two fluorine atoms are bonded to each carbon covalently and the geometry around the carbon atoms is trigonal planar.
Complete step by step answer:
The formula of Teflon is \[{{({{C}_{2}}{{F}_{4}})}_{n}}\]. The structure of Teflon is given below

Teflon is also known as PTFE or Polytetrafluoroethene. It is a thermoplastic polymer.
PTFE is manufactured by the addition polymerization of its monomers. It is the reaction which includes free radical polymerization reaction and includes the following steps - initiation, propagation and termination. Therefore, PTFE is an addition polymer.

Tetrafluoroetheneis a monomer of Teflon. The structure of tetrafluoroethene is given below

Therefore, the answer is – option (d) – None of these.
Additional Information:
Teflon is the plastic which has the lowest coefficient of friction. As we know, Teflon is very widely used as a non-stick coating for pans and other cookware. Also, being very unreactive Teflon is also very widely used in containers and pipework for reactive chemicals. It is also used as a material to provide resistance to heat & chemical attack. It is also used for making gaskets, pump packing, valves, oil seals, non-lubricated bearings, etc.
Note: The carbons in tetrafluoroethyleneare \[s{{p}^{2}}\] hybridized, where two fluorine atoms are bonded to each carbon covalently and the geometry around the carbon atoms is trigonal planar.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




