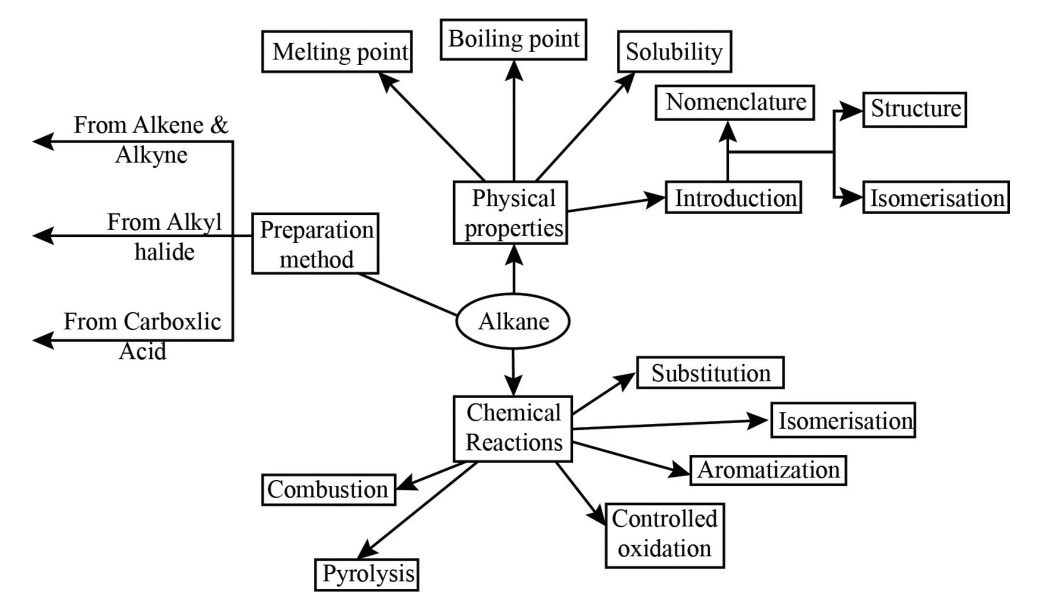
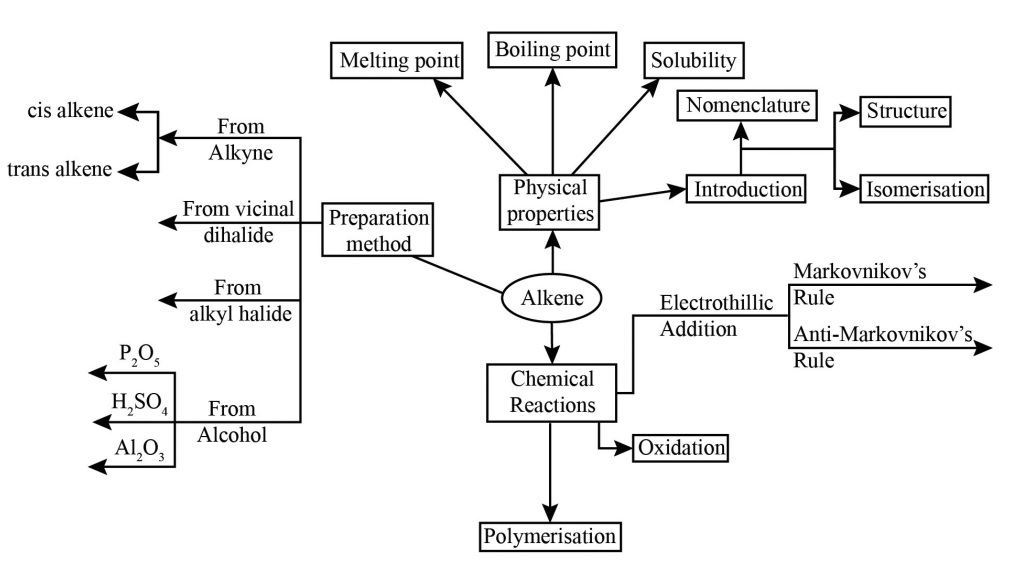
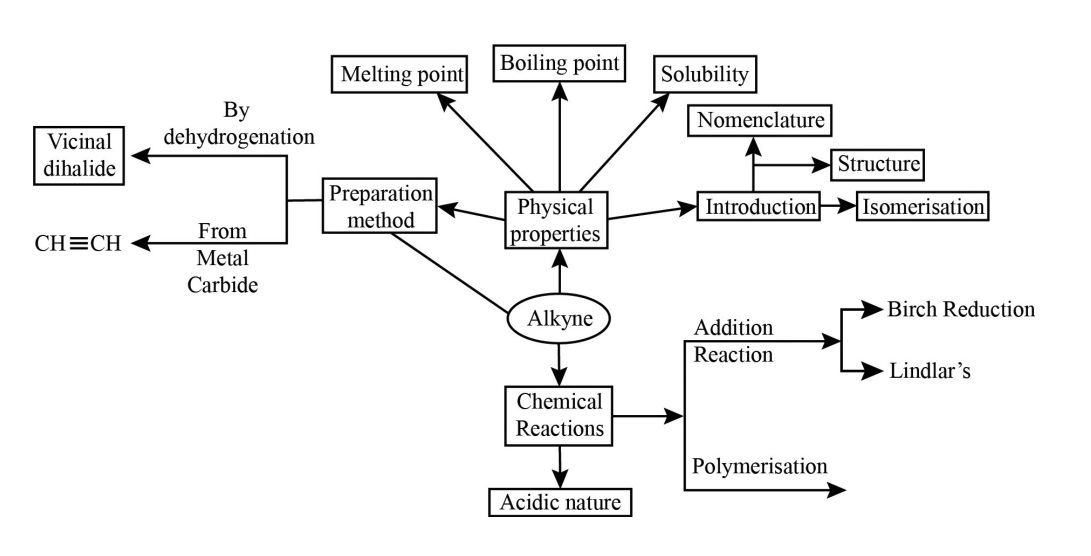
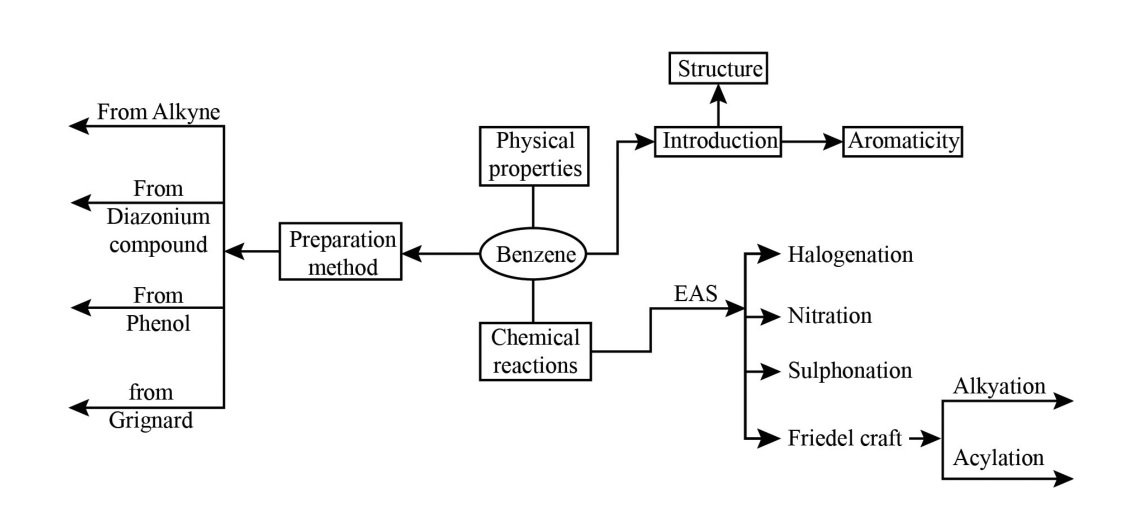
Hydrocarbons Class 11 NCERT PDF With Vedantu's Expert Solutions for FREE
FAQs on NCERT Solutions For Class 11 Chemistry Chapter 9 Hydrocarbons (2025-26)
1. How can I download the NCERT Solutions for Class 11 Chemistry Chapter 9 PDF in the CBSE 2025–26 format?
You can access and download the NCERT Solutions for Class 11 Chemistry Chapter 9 PDF, designed as per the updated CBSE 2025–26 syllabus, by visiting the official Vedantu website. These solutions strictly follow the NCERT pattern and are available for free in a stepwise, easy-to-understand format tailored for board preparation.
2. What is the correct stepwise method used to solve Exercise 9.3 in NCERT Class 11 Chemistry Chapter 9 Hydrocarbons?
To solve Exercise 9.3 of Class 11 Chemistry Chapter 9, start by carefully reading the question, identify the type of hydrocarbon or reaction involved, apply relevant chemical equations or mechanisms as guided in the NCERT textbook, and clearly show all steps such as balancing equations or drawing structural formulas in line with the official CBSE answer format.
3. Are these NCERT Solutions for Class 11 Chemistry Chapter 9 Hydrocarbons CBSE approved and suitable for board exams?
Yes, all answers provided for Class 11 Chemistry Chapter 9 strictly adhere to the latest CBSE guidelines for 2025–26 and are based on the official NCERT textbook, making them ideal for school exams, CBSE board assessments, and competitive exam foundation.
4. Where can I find stepwise answers to all intext questions of NCERT Class 11 Chemistry Chapter 9 Hydrocarbons?
Stepwise explanations for every intext question in Chapter 9, Hydrocarbons, are included in the Vedantu NCERT Solutions. These solutions provide accurate textbook answers in clear sequence, following the NCERT and CBSE 2025–26 format for maximum student benefit.
5. What is the NCERT answer key approach for multi-step reactions in Class 11 Chemistry Chapter 9?
The NCERT answer key for multi-step hydrocarbon reactions in Chapter 9 emphasizes breaking complex reactions into smaller, logical steps, showing each intermediate, and rationalizing the order of reagents or reaction conditions, exactly as per the NCERT exercise structure and CBSE marking scheme.
6. Which is the hardest chemistry chapter in class 11, and how does Chapter 9 compare in terms of NCERT exam difficulty?
Chapters like Thermodynamics and Equilibrium are generally considered the most challenging in Class 11 Chemistry. However, Chapter 9 Hydrocarbons is approachable when you follow systematic NCERT solutions, as the structure of stepwise answers reduces confusion for CBSE-style questions.
7. What is the official name of Class 11 Chemistry Chapter 9 as per the latest NCERT textbook?
According to the NCERT textbook for Class 11 Chemistry, Chapter 9 is titled "Hydrocarbons," covering structure, classification, preparation, and reactions of hydrocarbons following the CBSE syllabus for 2025–26.
8. How many chapters are there in Chemistry Class 11 NCERT, and where does Chapter 9 fit into the syllabus?
The Class 11 Chemistry NCERT consists of 14 chapters. Chapter 9 Hydrocarbons appears in the second part of the syllabus, focusing on organic chemistry fundamentals immediately after the chapter on Haloalkanes and Haloarenes, as per the CBSE order.
9. Is Class 11 Chemistry considered difficult, and do stepwise NCERT solutions for Chapter 9 help improve understanding?
Many students find Class 11 Chemistry tough due to new concepts and advanced problem-solving, but stepwise NCERT solutions for Chapter 9 Hydrocarbons help by offering structured, example-based answers that simplify complex organic reactions and mechanisms for CBSE exams.
10. Can I use these NCERT Solutions for Class 11 Chemistry Chapter 9 for JEE and NEET preparation along with CBSE board exams?
The NCERT Solutions for Class 11 Chemistry Chapter 9 are aligned with the core syllabus of CBSE, JEE, and NEET. Practicing these stepwise, NCERT pattern solutions helps build strong conceptual understanding and writing skills applicable in both board and entrance exams.
11. How can I avoid common mistakes when solving NCERT textbook Exercise 9.2 in Hydrocarbons?
To avoid errors in Exercise 9.2, always read the question carefully, follow the prescribed NCERT method for drawing structure or writing equations, check all intermediate steps, and cross-verify your answer with the official NCERT solution format before final submission.
12. Does the NCERT Solutions PDF include answers to both part 1 and part 2 of Class 11 Chemistry Chapter 9?
Yes, the downloadable NCERT Solutions PDF for Class 11 Chemistry Chapter 9 covers all exercises, including both part 1 and part 2 questions, providing a comprehensive stepwise solution for every problem from the official textbook.