
2-Methyl butane reacting with ${ Br }_{ 2 }$ in sunlight mainly gives:
(A.) 1-Bromo-2-methyl butane
(B.) 2-Bromo-2-methyl butane
(C.) 2-Bromo-3-methyl butane
(D.) 1-Bromo-3-methyl butane
Answer
595.2k+ views
Hint: You should know that 2-Methyl butane reacting with ${ Br }_{ 2 }$ in sunlight mainly gives 3-degree haloalkane as a product. Now try to figure out the answer accordingly.
Complete step by step solution:
Now, we will look at the stepwise answer to this question.
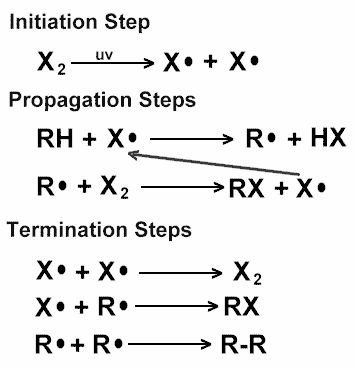
First, let’s see the mechanism of Chemical reaction involved in the Halogenation of alkane -
When alkane reacts with halogen in the presence of UV light, it gives free radical substitution reaction. Here is the complete mechanism for this reaction -
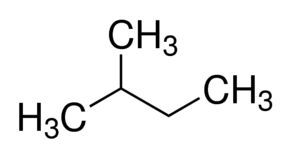
This is the structure of 2-Methyl butane -
Here Bromine gets substituted to hydrogen, where carbon has the least number of hydrogen atoms.
The carbon where -${ CH }_{ 3 }$ group is attached is three-degree carbon. Radical formed on this carbon will be stable so H-radical will be replaced with Br-radical. The final product will be 2-Bromo-2-methyl butane.
This is the complete reaction involved in this process -
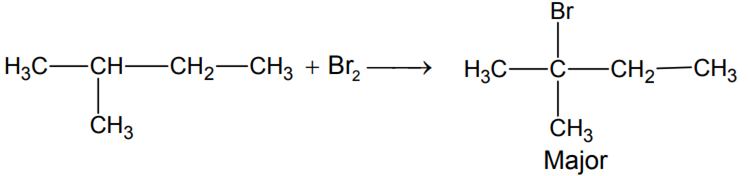
Option A) 1-bromo-2- methyl butane is incorrect.
Option B) 2-bromo-2- methyl butane is correct.
Option C) 2-bromo-3- methylbutane is incorrect.
Option D) 1-bromo-3- methylbutane is incorrect.
Therefore, we can conclude that the correct answer to this question is option B.
Note: We should also know that Alkanes are far less reactive than alkenes. They will only react with bromine water in the presence of UV light. As we have already discussed alkanes undergo substitution reactions with halogens under these conditions, and will slowly decolorize bromine water.
Complete step by step solution:
Now, we will look at the stepwise answer to this question.
First, let’s see the mechanism of Chemical reaction involved in the Halogenation of alkane -
When alkane reacts with halogen in the presence of UV light, it gives free radical substitution reaction. Here is the complete mechanism for this reaction -

This is the structure of 2-Methyl butane -

Here Bromine gets substituted to hydrogen, where carbon has the least number of hydrogen atoms.
The carbon where -${ CH }_{ 3 }$ group is attached is three-degree carbon. Radical formed on this carbon will be stable so H-radical will be replaced with Br-radical. The final product will be 2-Bromo-2-methyl butane.
This is the complete reaction involved in this process -
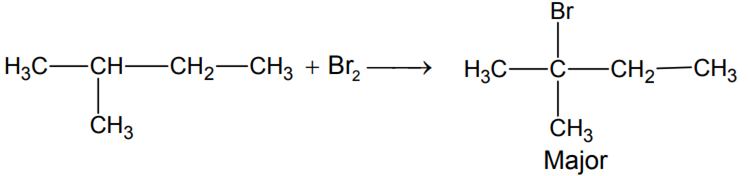
Option A) 1-bromo-2- methyl butane is incorrect.
Option B) 2-bromo-2- methyl butane is correct.
Option C) 2-bromo-3- methylbutane is incorrect.
Option D) 1-bromo-3- methylbutane is incorrect.
Therefore, we can conclude that the correct answer to this question is option B.
Note: We should also know that Alkanes are far less reactive than alkenes. They will only react with bromine water in the presence of UV light. As we have already discussed alkanes undergo substitution reactions with halogens under these conditions, and will slowly decolorize bromine water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




