
An element ‘X’ has valency of H. Write its formula for carbonate?
Answer
582.6k+ views
Hint: The capability of an atom to associate with other same or different atoms to form a molecule is known as its valency. The number of bonds that an atom forms with other atoms is going to depend on the valency of the particular atom.
Complete step by step answer:
- In the question it is given that an ‘X’ has valency of H.
- The valency of H means Hydrogen is 1 because hydrogen has only one electron in its electronic configuration in s orbital.
- The electronic configuration of Hydrogen is $1{{s}^{1}}$ .
- Means element ‘X’ also has a valency of one as per the given question.
- The molecular formula of carbonate ion is $CO_{3}^{2-}$ .
- We can see clearly that the carbonate has a charge of -2 in its molecular formula.
- Therefore carbonate ion reacts with 2 moles of ‘X’ and forms the product as shown in the below equation.
\[CO_{3}^{2-}+2X\to {{X}_{2}}C{{O}_{3}}\]
- Because ‘X’ has a valency of one the above reaction is possible.
- The chemical formula of ‘X’ with carbonate is ${{X}_{2}}C{{O}_{3}}$ .
Additional information:
- The structure of carbonate radical is as follows.
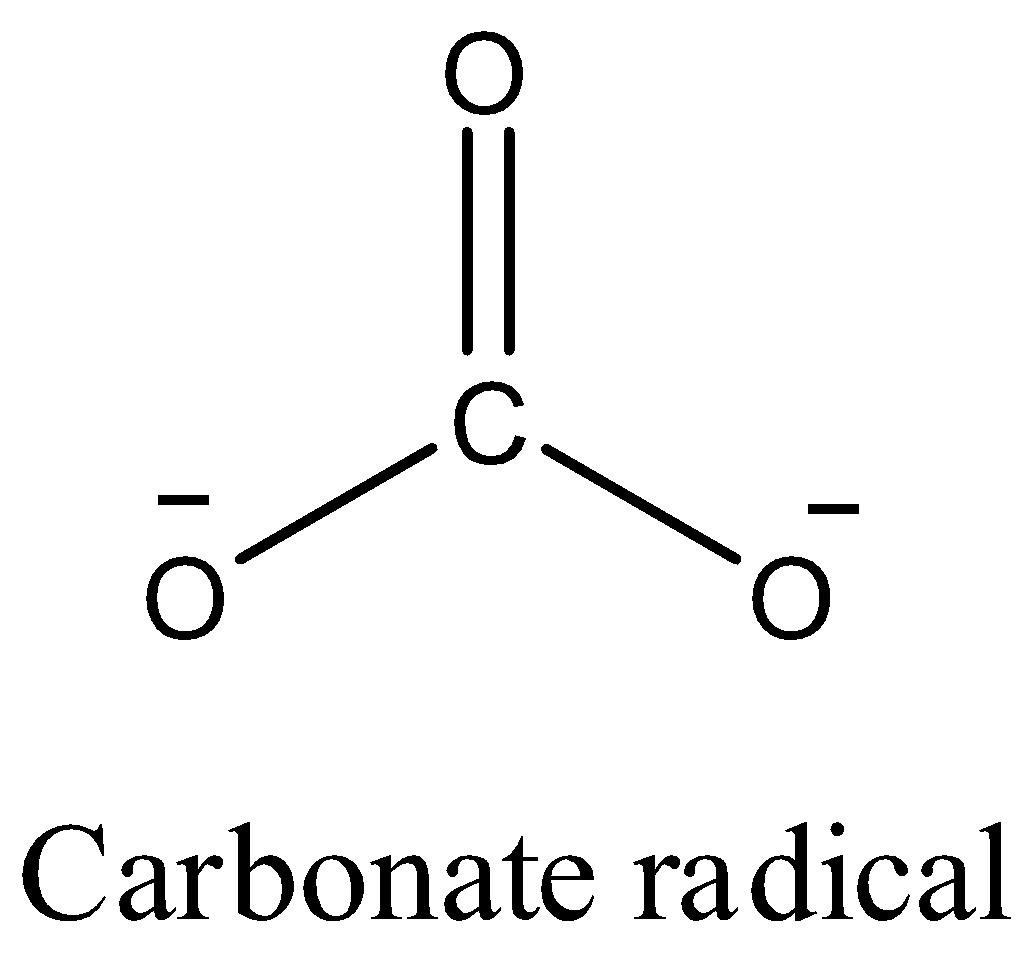
- We can see that the carbonate radical contains three oxygen atoms in its structure.
- One oxygen atom is bonded to carbon through a double bond and the remaining two oxygen atoms are bonded to carbon through single bond and contain a negative charge on them.
- Oxygen has one negative charge means it combines with one another atom.
- In the given compound there are two oxygen atoms having two negative charges means they combine with two other atoms to get neutralized.
Note: Carbonate radical combines easily with two hydrogen ions and forms carbonic acid. We can represent the carbonate radical as $CO_{3}^{2-}$ . The reaction of carbonate ion $CO_{3}^{2-}$ with two hydrogens ions as follows. \[CO_{3}^{2-}+2{{H}^{+}}\to \underset{Carbonic\text{ }acid}{\mathop{{{H}_{2}}C{{O}_{3}}}}\,\]
The valency of bicarbonate ions is one.
Complete step by step answer:
- In the question it is given that an ‘X’ has valency of H.
- The valency of H means Hydrogen is 1 because hydrogen has only one electron in its electronic configuration in s orbital.
- The electronic configuration of Hydrogen is $1{{s}^{1}}$ .
- Means element ‘X’ also has a valency of one as per the given question.
- The molecular formula of carbonate ion is $CO_{3}^{2-}$ .
- We can see clearly that the carbonate has a charge of -2 in its molecular formula.
- Therefore carbonate ion reacts with 2 moles of ‘X’ and forms the product as shown in the below equation.
\[CO_{3}^{2-}+2X\to {{X}_{2}}C{{O}_{3}}\]
- Because ‘X’ has a valency of one the above reaction is possible.
- The chemical formula of ‘X’ with carbonate is ${{X}_{2}}C{{O}_{3}}$ .
Additional information:
- The structure of carbonate radical is as follows.

- We can see that the carbonate radical contains three oxygen atoms in its structure.
- One oxygen atom is bonded to carbon through a double bond and the remaining two oxygen atoms are bonded to carbon through single bond and contain a negative charge on them.
- Oxygen has one negative charge means it combines with one another atom.
- In the given compound there are two oxygen atoms having two negative charges means they combine with two other atoms to get neutralized.
Note: Carbonate radical combines easily with two hydrogen ions and forms carbonic acid. We can represent the carbonate radical as $CO_{3}^{2-}$ . The reaction of carbonate ion $CO_{3}^{2-}$ with two hydrogens ions as follows. \[CO_{3}^{2-}+2{{H}^{+}}\to \underset{Carbonic\text{ }acid}{\mathop{{{H}_{2}}C{{O}_{3}}}}\,\]
The valency of bicarbonate ions is one.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




