
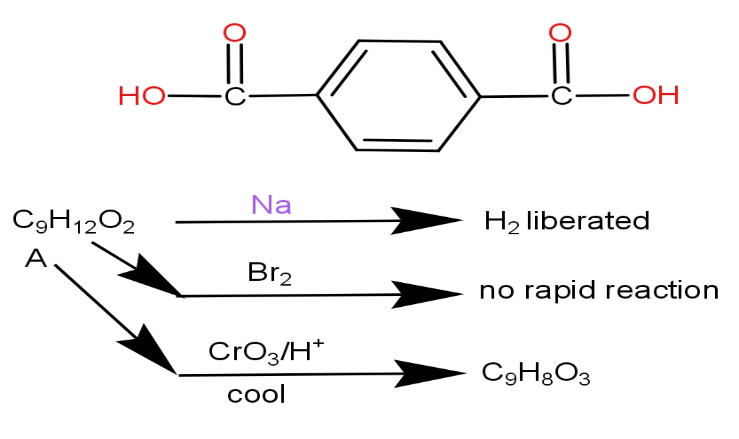
An optically active alcohol of formula ${C_9}{H_{12}}{O_2}$produced the following compound when refluxed with $KMn{O_4}$. The original compound showed properties as shown. What is the structure of (A) ?
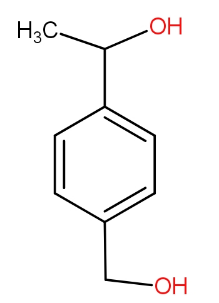
(A)
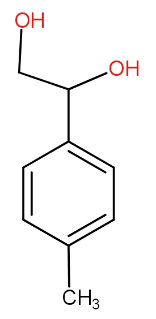
(B)
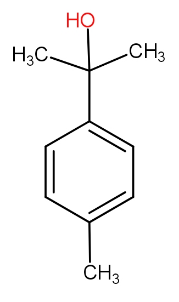
(C)
(D) Both (A) and (B)




Answer
577.2k+ views
Hint: $KMn{O_4}$ performs the oxidation of alkenes to benzoic acids of those compounds which have alpha hydrogen attached to benzene carbon. So, the answer to this would be the compounds which have primary or secondary carbon atoms attached to the benzene ring.
Complete step by step solution:
First, let us know what are optically active compounds.
The optically active compounds are those that can rotate the plane polarised light. One enantiomer rotates the plane polarised light right while the other one rotates it to left.
Now, let us see the use of $KMn{O_4}$. It functions in performing the oxidation of aromatic alkanes to benzoic acid. But it requires one condition. This requires alpha hydrogen attached to benzene carbon.
So, the compound in the option that will contain the alpha hydrogen attached with benzene carbon will be the correct answer.
If we see the first option, it has alpha hydrogen attached with benzene carbon. So, it can be the correct answer.
The compound in the second option also has the alpha hydrogen attached with benzene carbon. So, even this can be the answer.
The third option compound does not have alpha hydrogen attached with benzene carbon. So, this will be eliminated.
Further, it is said that the compound showed some other properties.
Both the compounds in option (A) and (B) release hydrogen on reaction with sodium metal.
The second characteristic is that there is no rapid reaction with bromine gas. We know that the function of bromine gas is to do an additional reaction and benzene does not easily give an additional reaction. So, even both the compounds show this property.
Even the third property is shown by both these molecules.
So, the correct answer will be option (D).
Note: It must be noted that the benzene ring is electron-rich. If it gives an additional reaction, then its resonance breaks and the stability is lost. So, it does not give an additional reaction very easily.
Further, $Cr{O_3}/{H^ + }$ reacts with primary alcohol to give carboxylic acid while it reacts with secondary alcohol to give ketones. But it does not react with tertiary alcohol.
Complete step by step solution:
First, let us know what are optically active compounds.
The optically active compounds are those that can rotate the plane polarised light. One enantiomer rotates the plane polarised light right while the other one rotates it to left.
Now, let us see the use of $KMn{O_4}$. It functions in performing the oxidation of aromatic alkanes to benzoic acid. But it requires one condition. This requires alpha hydrogen attached to benzene carbon.
So, the compound in the option that will contain the alpha hydrogen attached with benzene carbon will be the correct answer.
If we see the first option, it has alpha hydrogen attached with benzene carbon. So, it can be the correct answer.
The compound in the second option also has the alpha hydrogen attached with benzene carbon. So, even this can be the answer.
The third option compound does not have alpha hydrogen attached with benzene carbon. So, this will be eliminated.
Further, it is said that the compound showed some other properties.
Both the compounds in option (A) and (B) release hydrogen on reaction with sodium metal.
The second characteristic is that there is no rapid reaction with bromine gas. We know that the function of bromine gas is to do an additional reaction and benzene does not easily give an additional reaction. So, even both the compounds show this property.
Even the third property is shown by both these molecules.
So, the correct answer will be option (D).
Note: It must be noted that the benzene ring is electron-rich. If it gives an additional reaction, then its resonance breaks and the stability is lost. So, it does not give an additional reaction very easily.
Further, $Cr{O_3}/{H^ + }$ reacts with primary alcohol to give carboxylic acid while it reacts with secondary alcohol to give ketones. But it does not react with tertiary alcohol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




